Regulatory Solutions
MedBoard platform,
it is all it takes to transform your
Regulatory Intelligence and Operations.
225+ Countries Covered
15+ Regulatory Areas
Powerful Software Solutions
Solutions for each step of an effective Regulatory System
in a unified platform that provides end-to-end data management
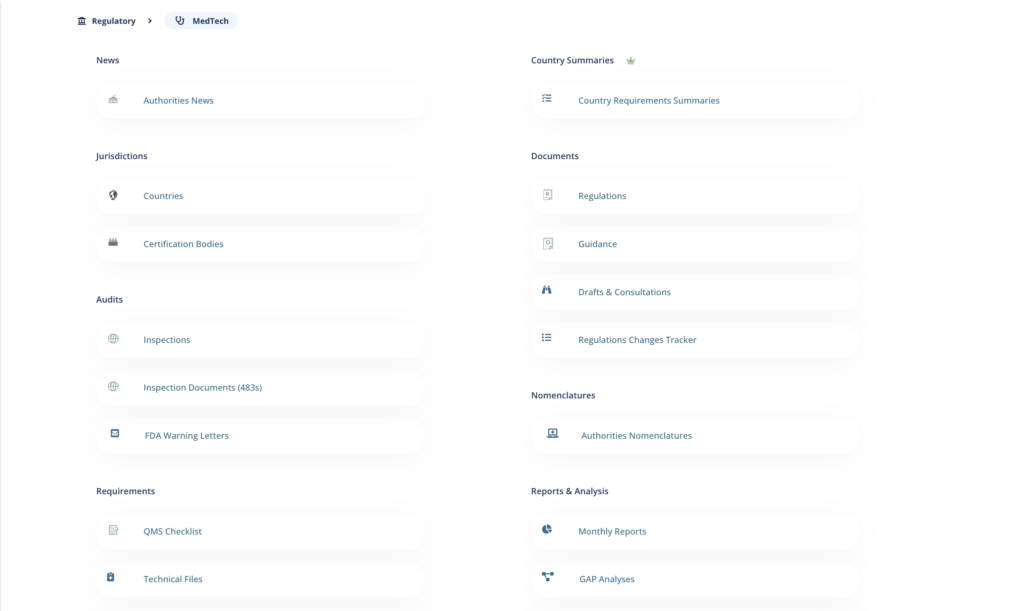
Global regulatory data access
MedBoard regulatory data in 225+ Countries and 15+ Regulatory Areas. Access to up to date Regulatory Intelligence, data, tools and news, organized, classified and curated by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma, BioTech and Clinical Trials, including countries regulatory summaries for an unparalleled research and intelligence, so you can focus on what truly matters. Resources include:
- Real-time Authorities News
- Real-time Standards Updates and Adoptions
- 150k+ Countries Summaries Requirements
- Countries Profiles
- Documents, translations and databases
- Resources, flowcharts and tools
Continuous & Instant Access to Trusted Information, Data & Knowledge.
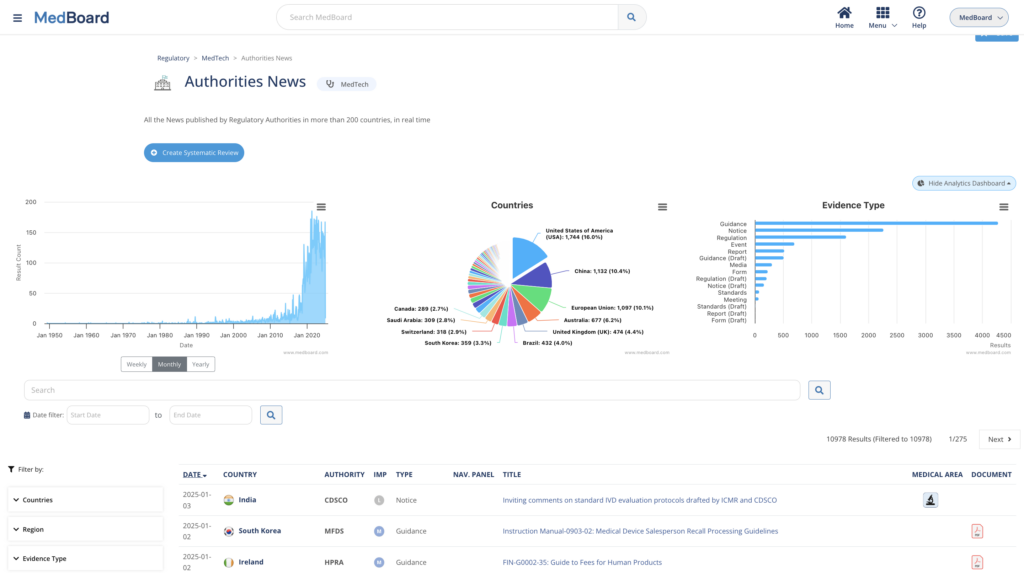
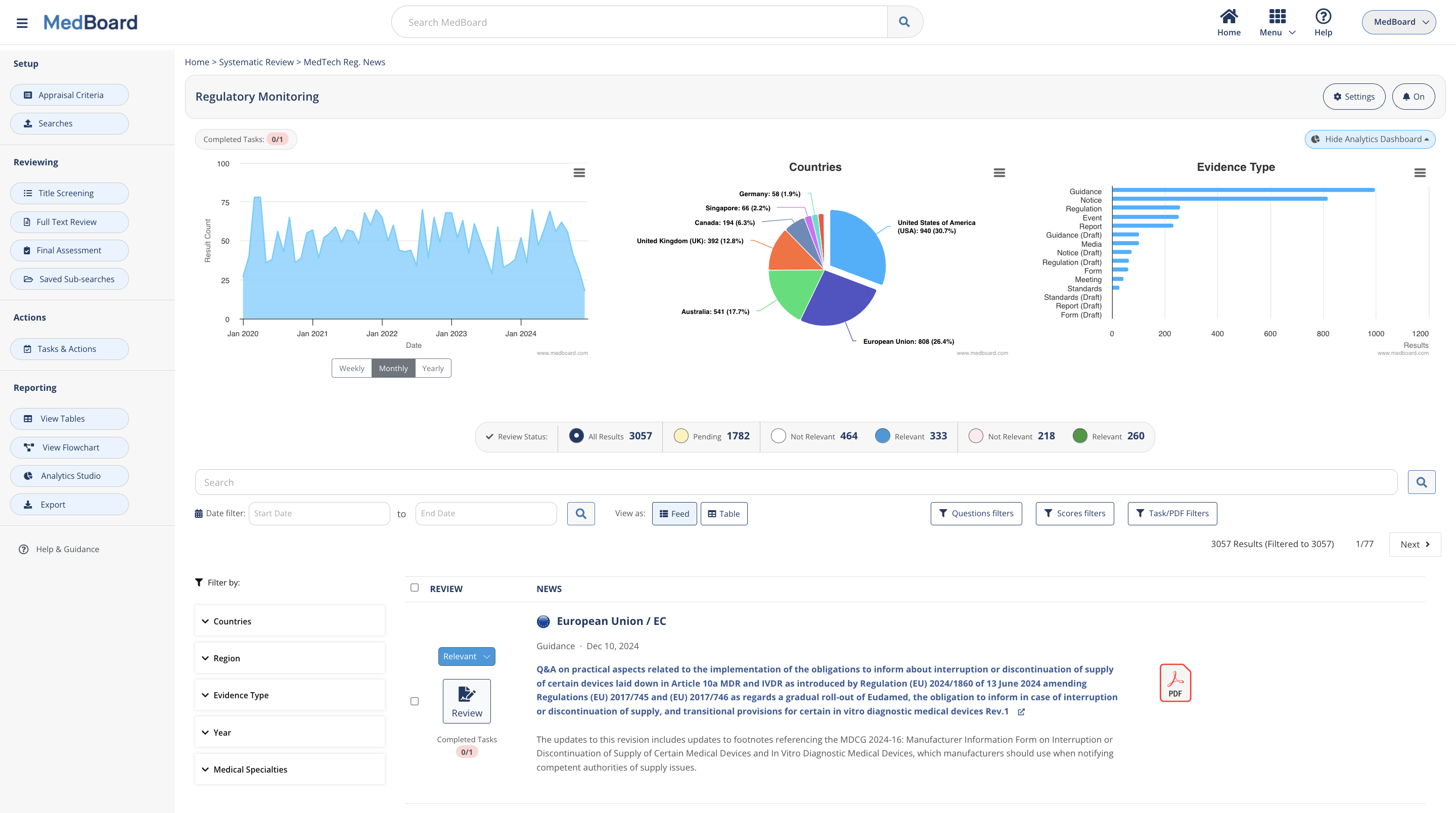
Review systematically regulatory news
Customize your scope with the countries and regulatory areas applicable to you, and perform proactive Regulatory Monitoring, Changes Impact Assessment, and Reporting in a systematic, easy, and integrated process. Features include:
- Real time updates and notifications
- Appraisal process for a consistent review framework to assess impact
- AI support for data collection and extraction
- A collaborative process for seamless execution by the team
- Ready to use reporting tools
Continuous monitoring, implementation and reporting.
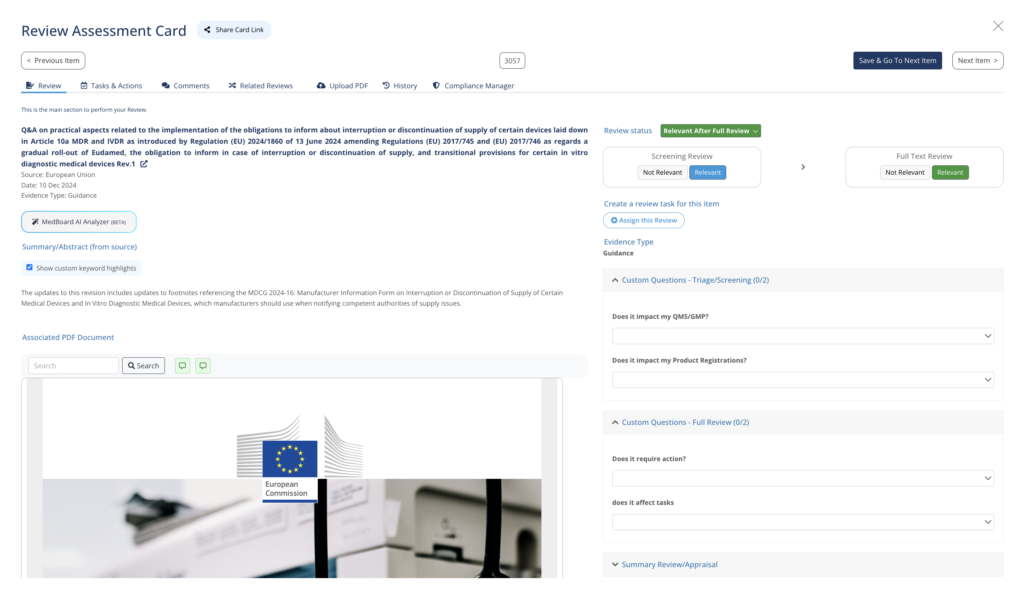
Keep your compliance scope under control
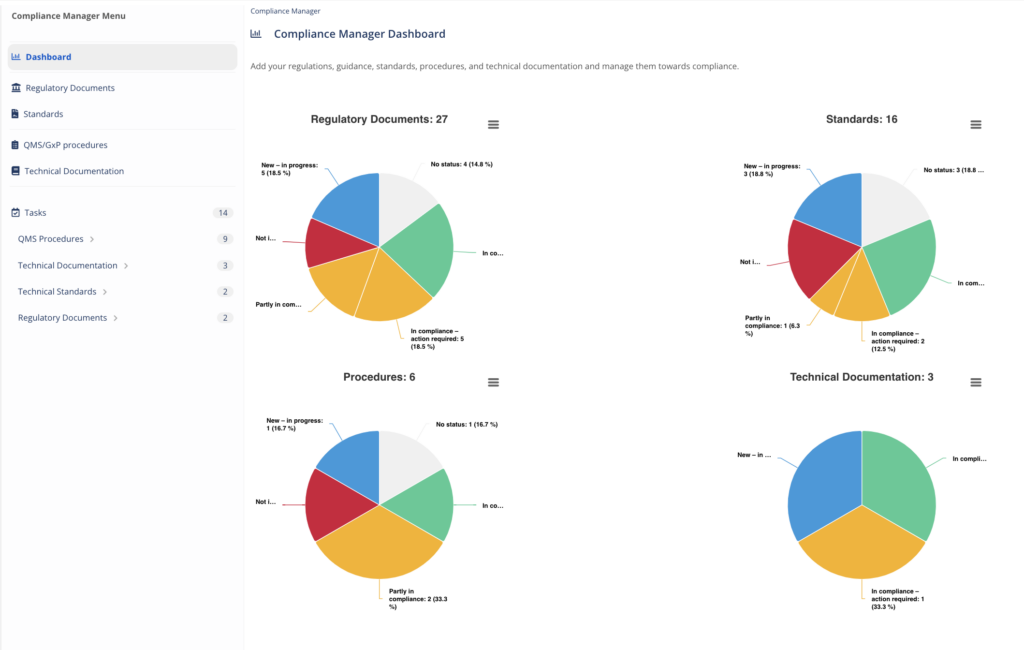
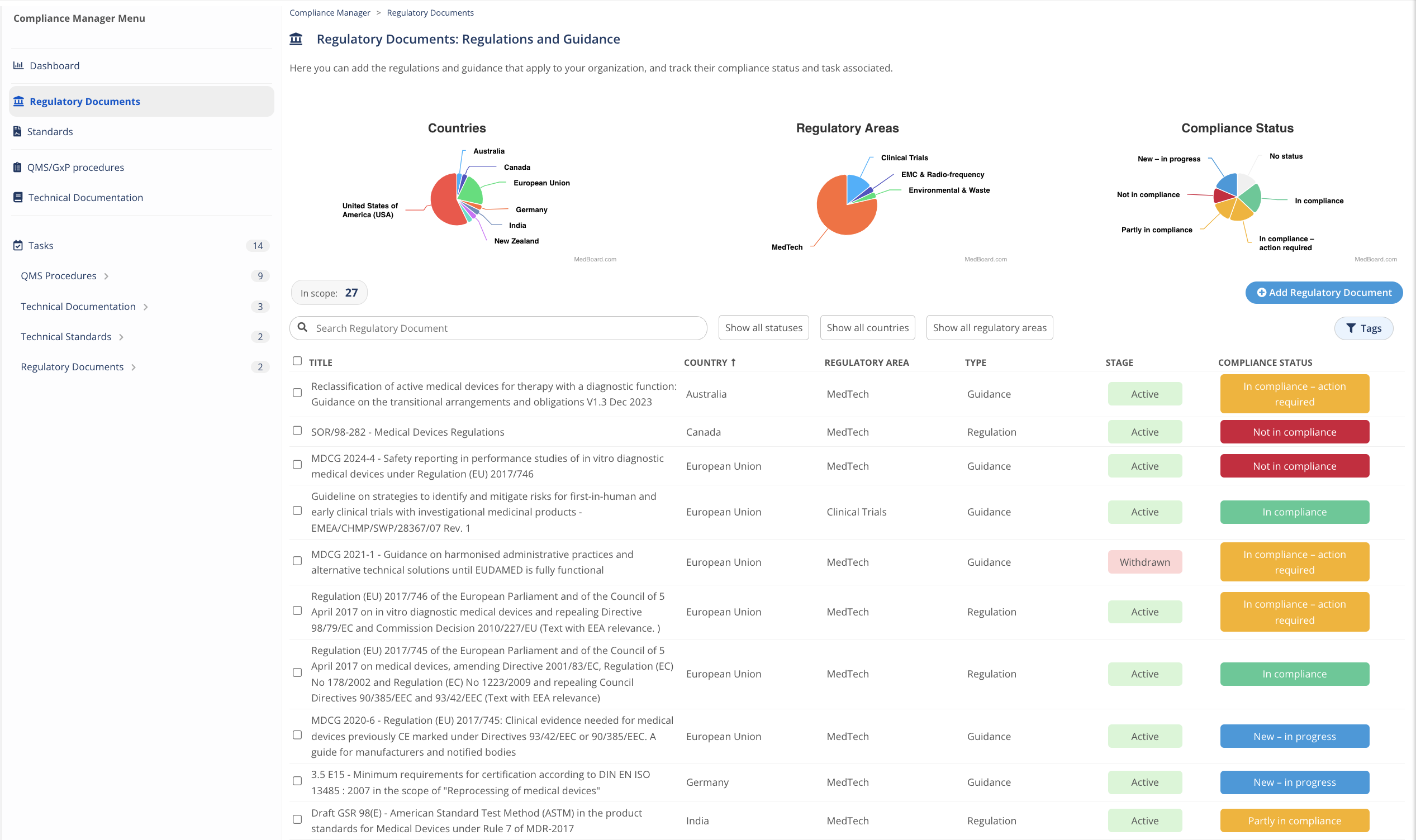
Organize, manage and track your compliance evidence with the Compliance Manager. This module helps to identify and manage the specific:
- Regulations, guidance and regulatory documents that apply to you
- Technical standards within your scope
- Technical Documentation
- Procedures and processes
Keep the big picture and understand your compliance status any time. Powered by MedBoard Intelligence to keep up to date with changes and requirements.
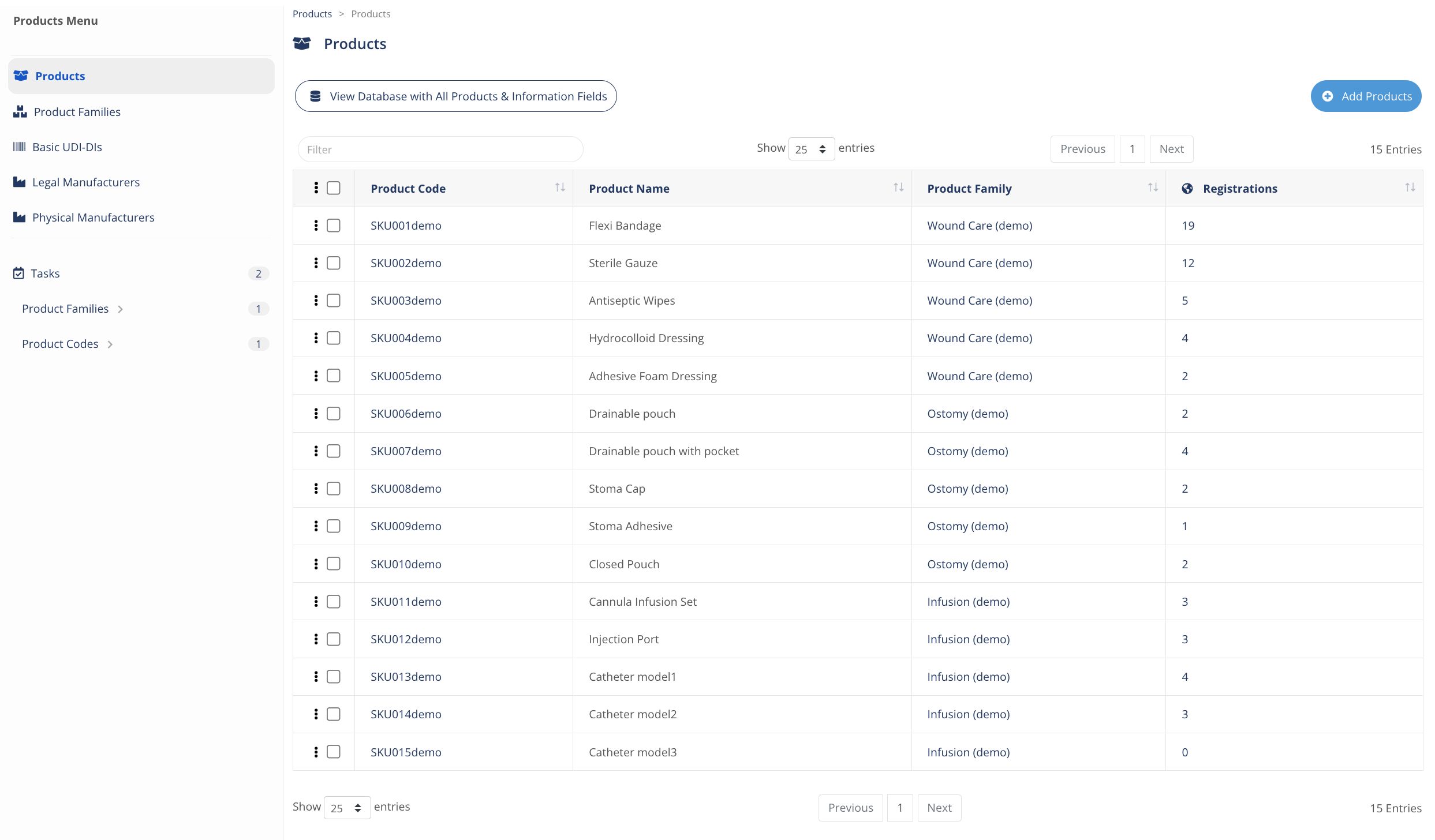
Manage your Products Information
A powerful ready-to-use Products Information Management to build, manage and track information about your products, product codes, SKUs, and its information, including unique identifiers (e.g. UDI) and claims.
- Integrations to link your products with evidence, compliance, registrations and many more.
- Audit Trail: ready to export data and dashboards to report information any time.
An essential regulatory Data Management solution for any organization.
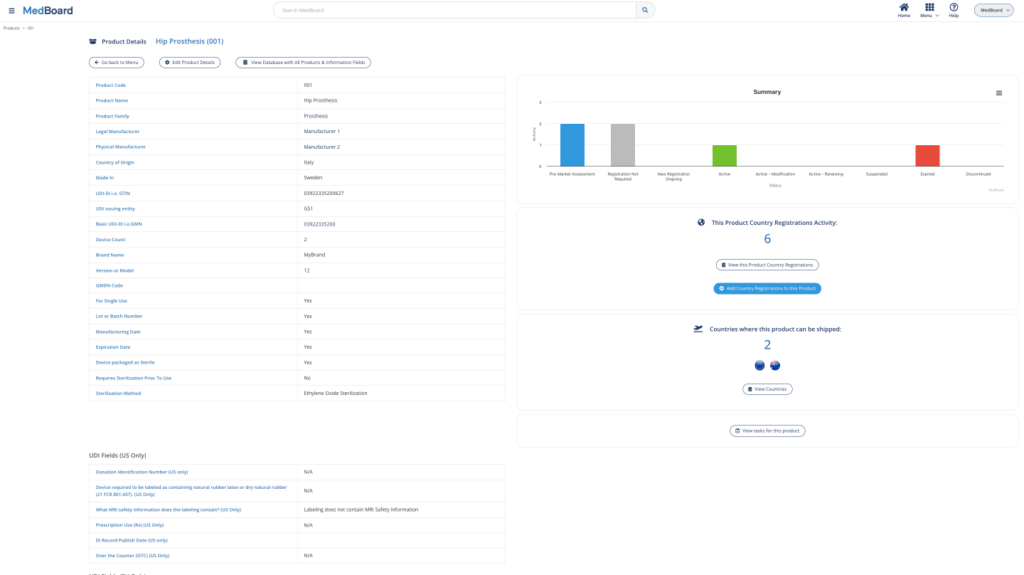
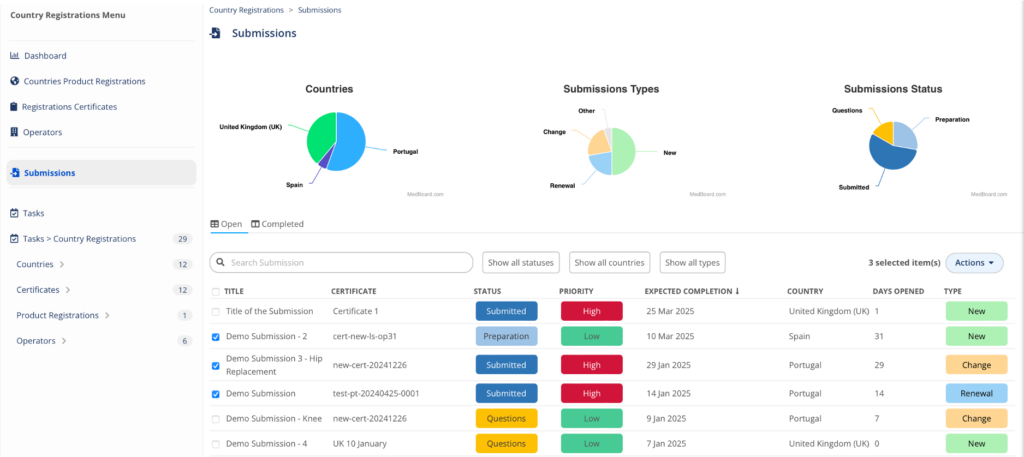
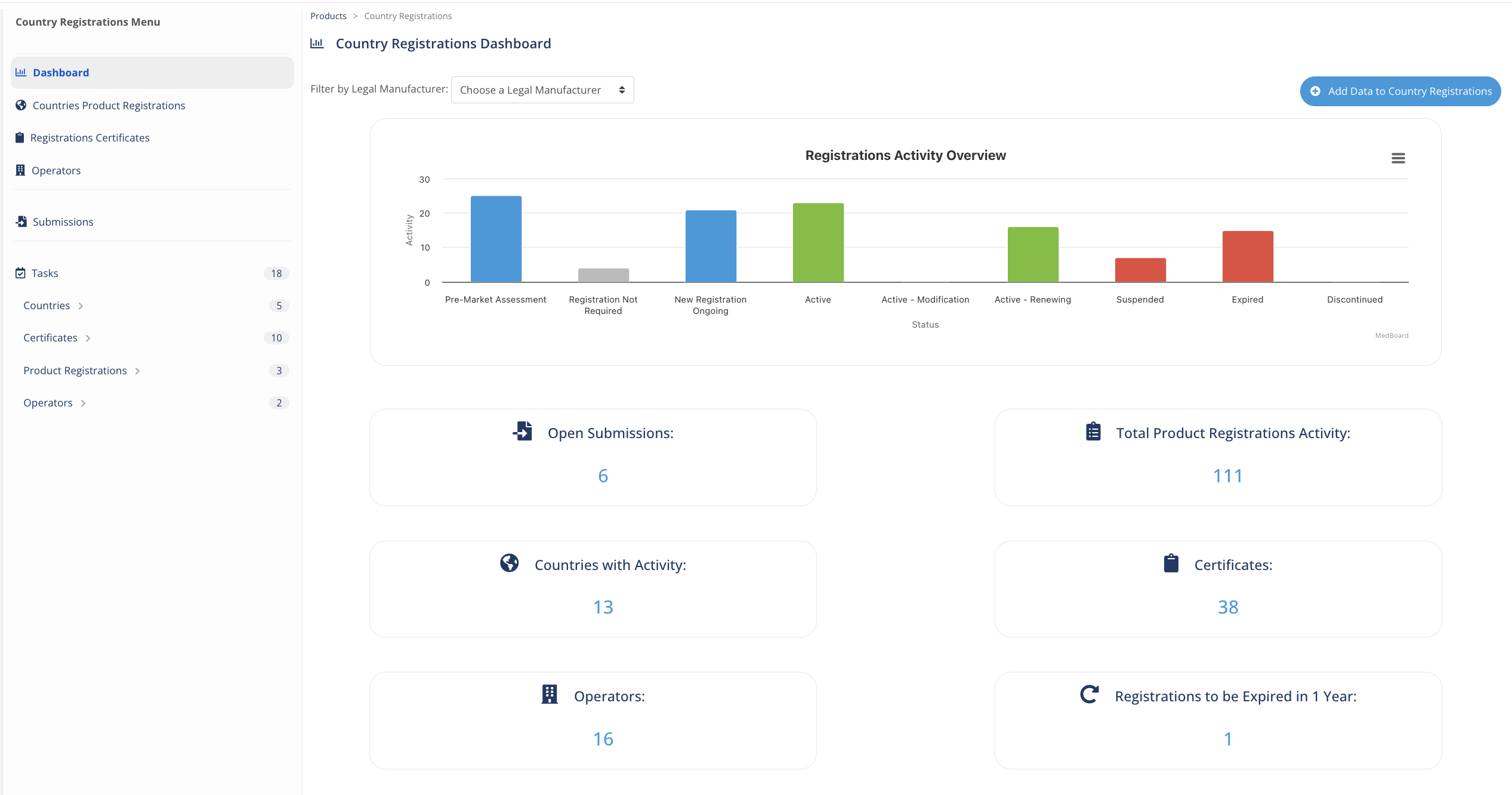
Manage your Country Registrations
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, submissions, licenses and economic operators, all integrated together with other MedBoard solutions and intelligence. Easily manage:
- Registrations
- Certificates
- Operators
- Submissions
- Renewals, tasks, documents and many more
An essential regulatory Data Management solution for any organization.
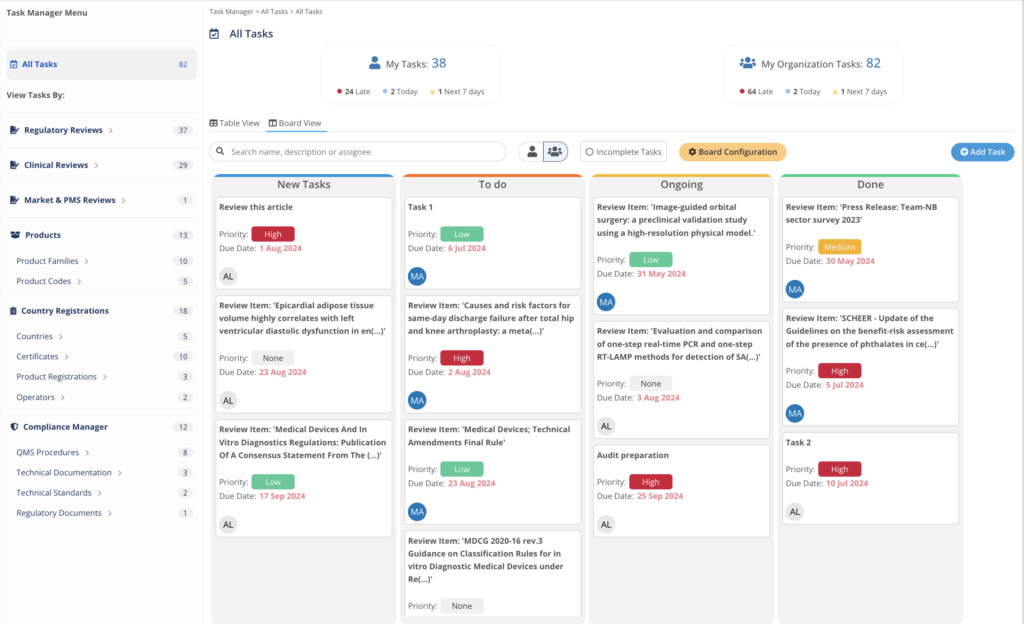
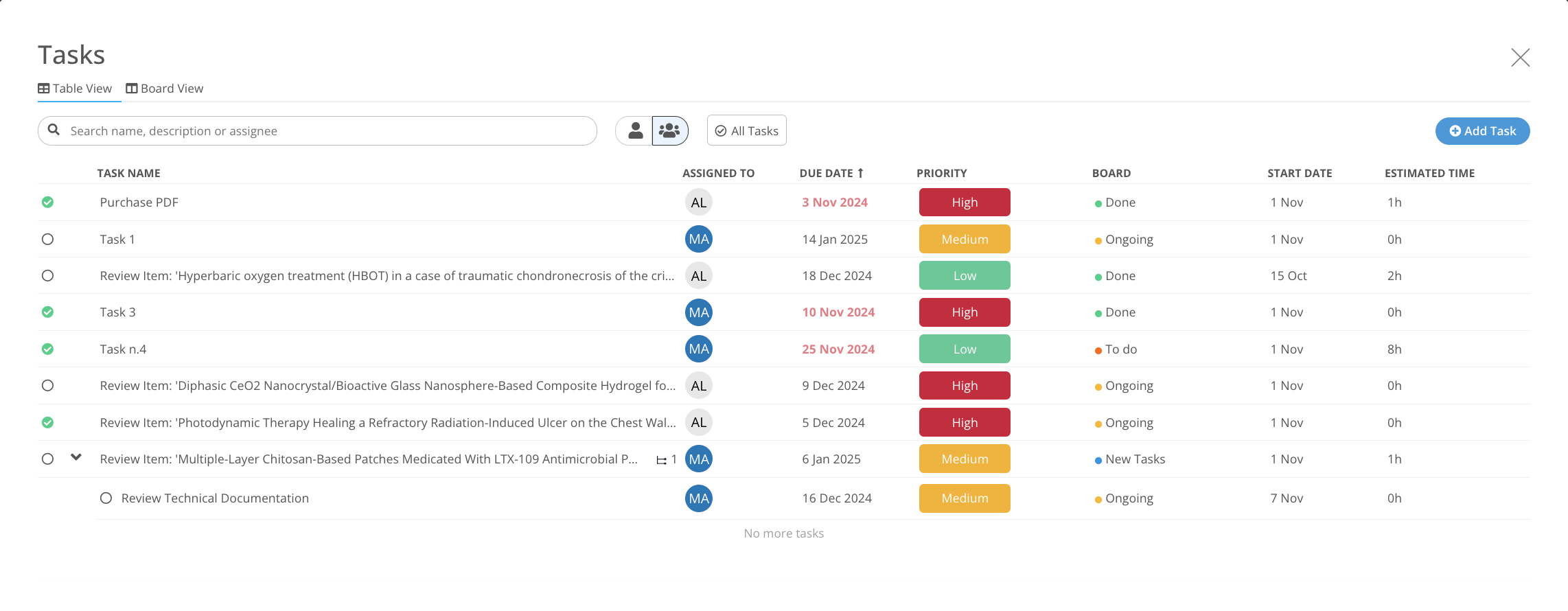
Manage work and implementation within every process
Task Manager integrates with each regulatory MedBoard solution for actions and implementation of tasks. Keep control on deliverables and
- Create Tasks, Actions linked to Regulatory Updates
- Deadlines and Responsible Person
- Email Notifications and Alerts
- Dashboard to report progress and risks
Actions, implementation, and planning managed easily from one single place.
100%
CSAT
Customer Satisfaction (CSAT) Score,
with an average overall satisfaction with MedBoard of 4,8/5.
50%
Reduced Manual Work
86% of MedBoard users report reducing
by more than 50% the need for
manual actions and work.
3x-10x
Faster Processes
78% of MedBoard users reported
performing actions 3x to 10x faster,
accelerating their processes.
Go Beyond: MedBoard platform offers even more
Discover all our connected solutions
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your evidence transformation journey






