
Regulatory News Systematic Reviews
Perform proactive regulatory monitoring and impact assessments in a systematic, easy, and integrated process.
Customize 225+ Countries and 15+ Regulatory areas to your needs. Use MedBoard software, automations, workflows, integrated intelligence, and AI-powered features, to produce compliant regulatory reviews faster, with more precision and more cost-effective.
Real Time
Powered by MedBoard Global Data, get automatic updates and notifications in real-time from Authorities News based on your criteria.
Customizable
Customize your review framework for a consistent process shaped to your needs. Apply systematic reviews to many databases.
Connected
Connected with other MedBoard solutions such as Intelligence, Compliance Manager, Products Information or Countries Registrations.
Audit Trail
Show the evidence that you keep track and review updates, whether it is for internal use, customers, or for Authorities.
Easy to Use
Connected with other MedBoard solutions such as Compliance Manager, Products Information or Countries Registrations.
Secure
Your privacy and security are our top priority. MedBoard is ISO 27001 certified, ensuring robust information security management.
Say Goodbye to manual processes
Features that automate your workflow
and minimize your manual work
Customize your monitoring and appraisal
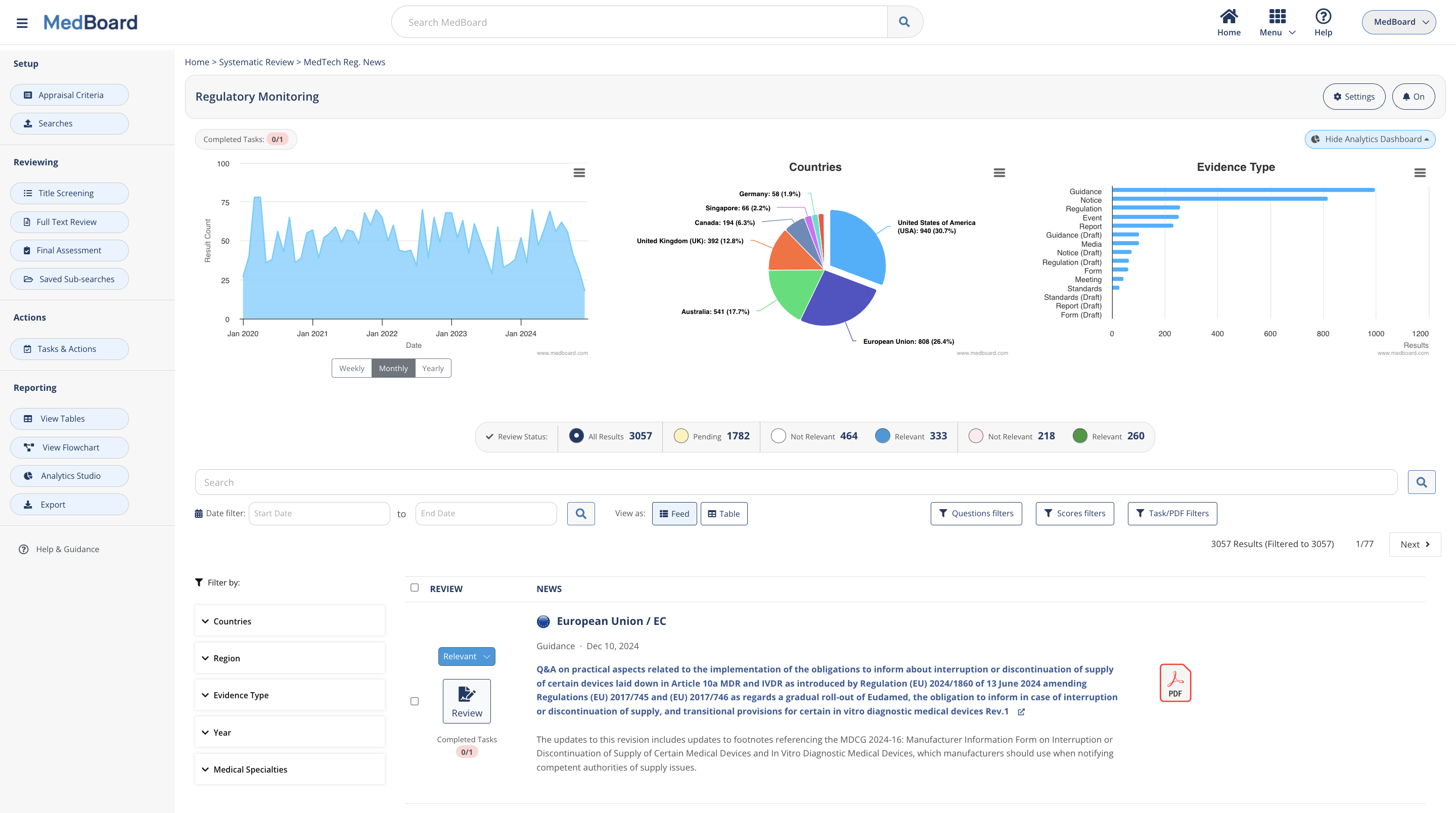
Authorities’ News and Standards Updates
Customize MedBoard real-time regulatory databases to your needs, including Authorities News and Standards Lifecycle Updates and Adoptions.
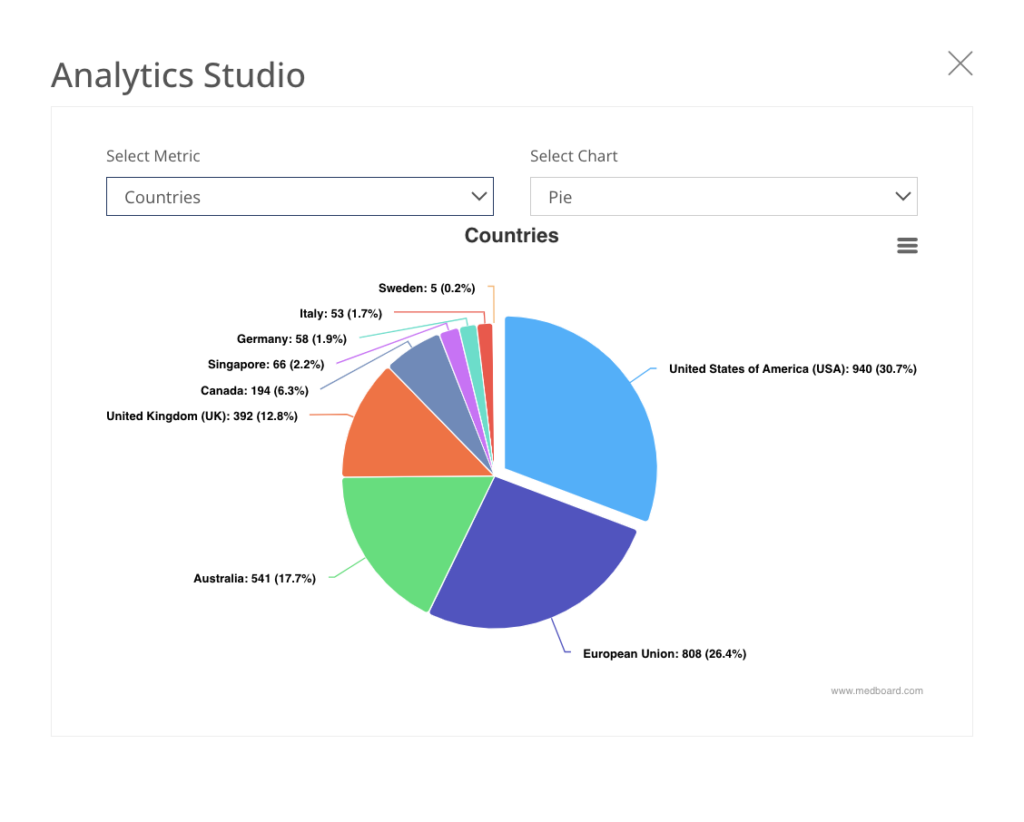
225+ Countries
Select the countries that apply to you and use filters and queries.
15+ Regulatory Areas
Select the sectors that apply to you, including MedTech, Pharma, Clinical Trials, AI, and many more.
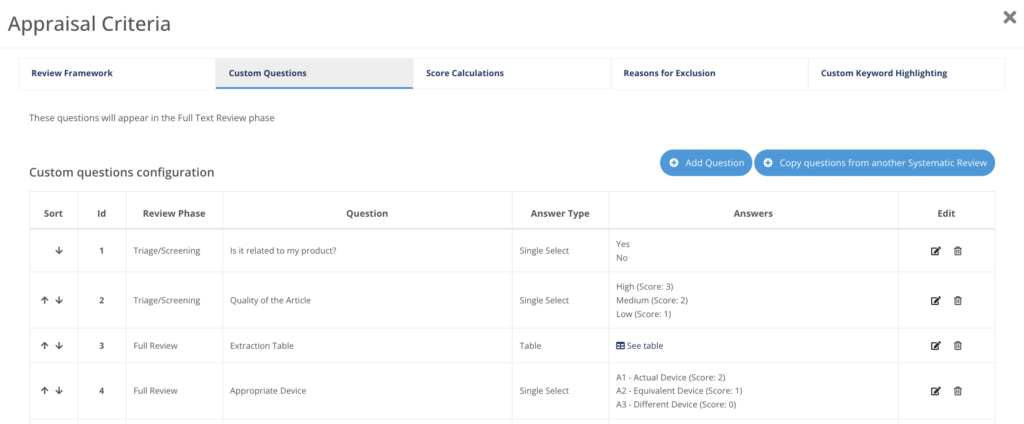
Configure your Appraisal Criteria
Fully customizable appraisal plan for Impact Assessments with custom questions, scores and extraction tables, adapted to your needs.
Changes evaluation, impact assessments and actions
Email Notifications
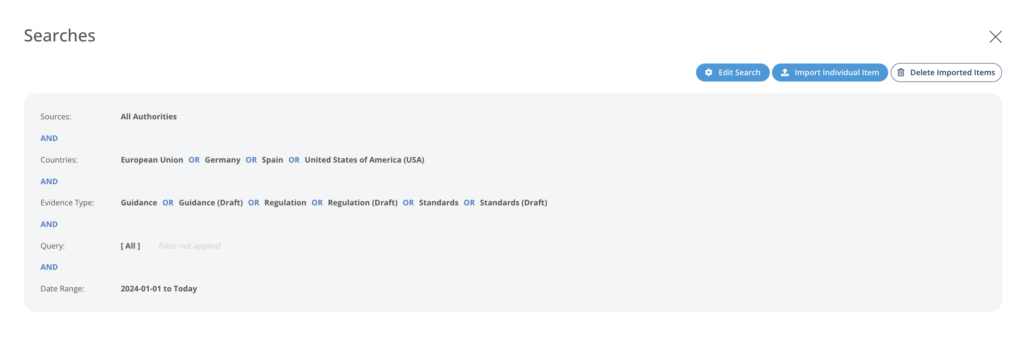
Get automatic updates and notifications in real-time from Authorities News based on your search plan and strategy.
Fast Screening
One-click screening on an easy to use interface supported by filters, sub-searches, custom filters, and many more features.
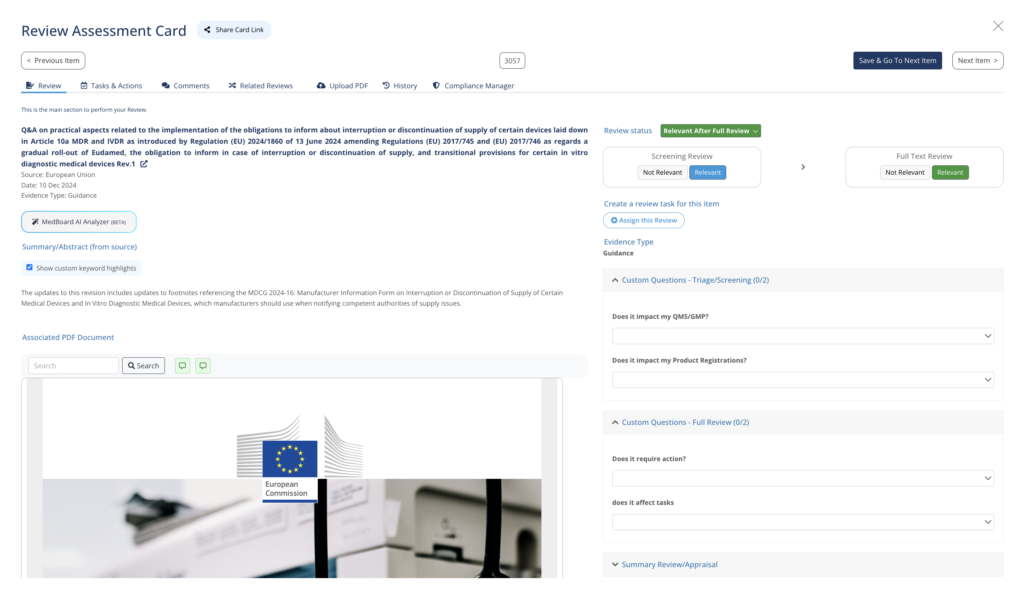
Full Review and Impact Assessment
Embedded Review Assessment Card for each update, side-by-side PDF viewer, custom questions and many more features.
MedBoard AI Analyzer
Use the MedBoard AI Analyzer to extract from regulatory documents key information, regulatory requirements, summaries, and documents cited.
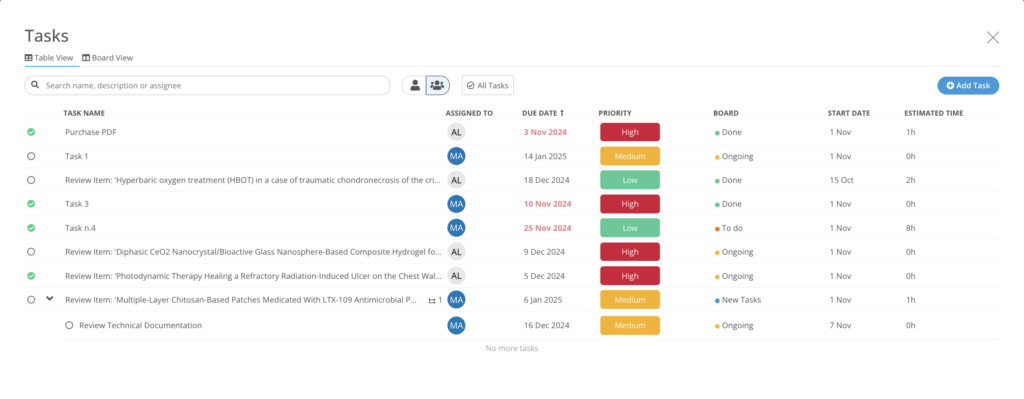
Task Manager
Ensure implementation with an integrated project management solution to assign reviews, collaborate, and create tasks, actions and deadlines.
Reporting
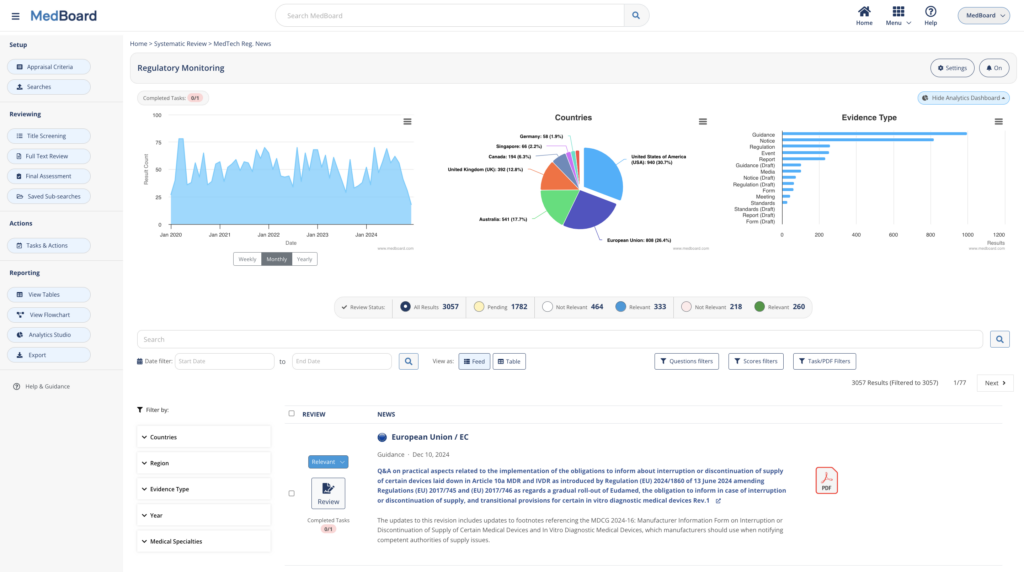
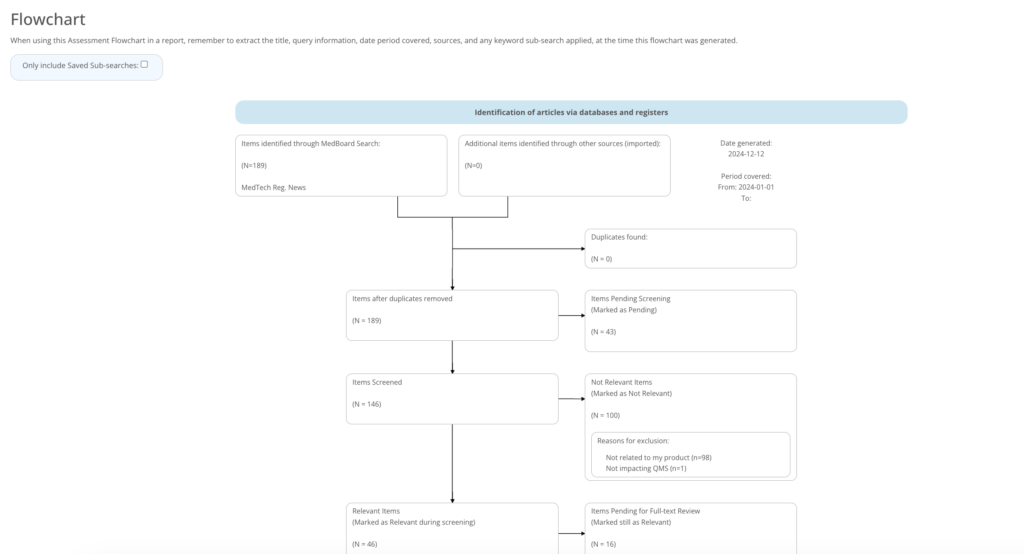
Report your reviews
Report instantly the outcomes of your review within a period, relevant items impacting the company, review progress with visual flowcharts, and impact assessments evaluations.
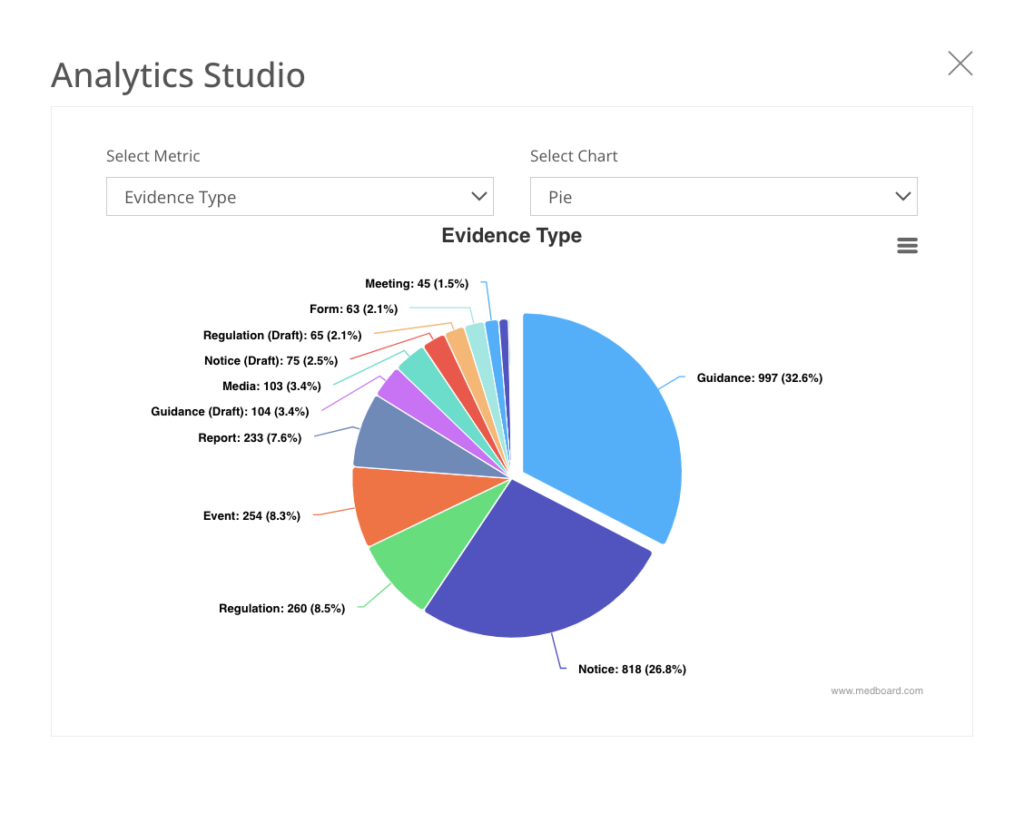
Export data, appraisals and analytics
Export data, flowcharts, appraisals and analytics linked to your review. Our reporting options are fully customizable and adapt to any filter selection.
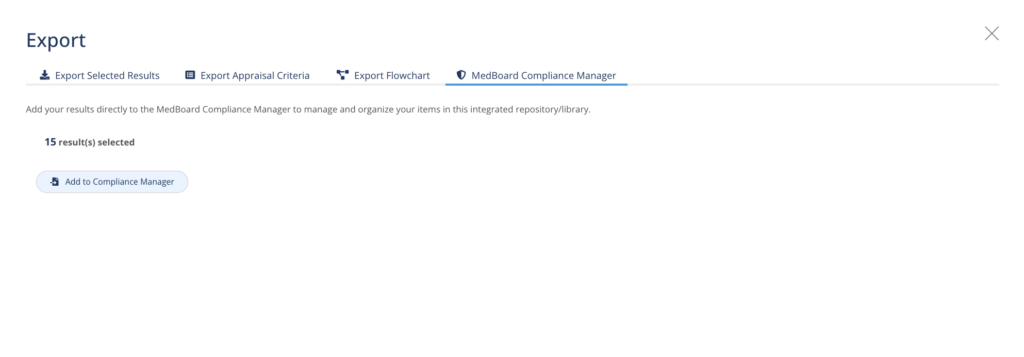
Transfer regulatory items to Compliance Manager automatically
Seamlessly integrated with the MedBoard Compliance Manager, transfer relevant documents directly from your Systematic Review and add them to your compliance scope.
100%
CSAT
Customer Satisfaction (CSAT) Score,
with an average overall satisfaction with MedBoard of 4,8/5.
50%
Reduced Manual Work
86% of MedBoard users report reducing
by more than 50% the need for
manual actions and work.
3x-10x
Faster Processes
78% of MedBoard users reported
performing actions 3x to 10x faster,
accelerating their processes.
Customer Stories
GUERBET – Pharmaceutical Manufacturer
“ I have found its Regulatory Systematic Reviews process to be easily incorporated into our existing procedures. It is also very helpful for Regulatory preparation for Management Reviews. “
Make the Most out of MedBoard
Discover our Software Products for Regulatory Needs and Professionals
![]()
Access to up to date Regulatory Intelligence, data, tools and News in more than 225 Countries, organized, curated and cleaned by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma/BioTech and Clinical trials, that includes countries curated summaries for an unparallel research and intelligence.
Add Documents and Standard that apply to your organization to the Compliance Manager!
![]()
Review systematically Regulatory News and Updates connected to our Global Data covering 225+ countries and 15+ regulatory areas in real time. Customize it, create protocols, impact assessments, actions, and reports easily with MedBoard.
![]()
Organize, manage and track your compliance evidence with the Compliance Manager. This module helps to identify and control the specific regulations, guidance, standards, procedures, technical documentation that apply to you, and to organize, action, and track your related compliance evidence.
![]()
A powerful ready-to-use Products Information Management to organize, manage and track information about your products, product codes, SKUs, and its information, including Unique Identifiers (e.g. UDI), all integrated together with Regulatory Intelligence and MedBoard Search.
![]()
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, licenses and economic operators, all integrated together with Regulatory Intelligence, Regulatory Reviews, Task Manager and MedBoard Search.
All modules integrate seamlessly with each other,
to keep all connected, in one place.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams
