
Products Information
Manage, organize and access information about your products, with this scalable, customizable, and secure solution.
Purposefully built for the life sciences organizations, with multiple fields and search capabilities, allows you to manage all your products information in one single place.
Integrated
Seamlessly integrated with MedBoard Country Registrations RIMS and other MedBoard regulatory and clinical solutions.
Scalable
Easy to use, flexible and scalable system to manage products information and multiple fields.
Secure
Your Privacy and Security are our top priority. MedBoard is ISO 27001 certified, ensuring information security.
Powerful features and automations
for Products Information Management
Manage products data
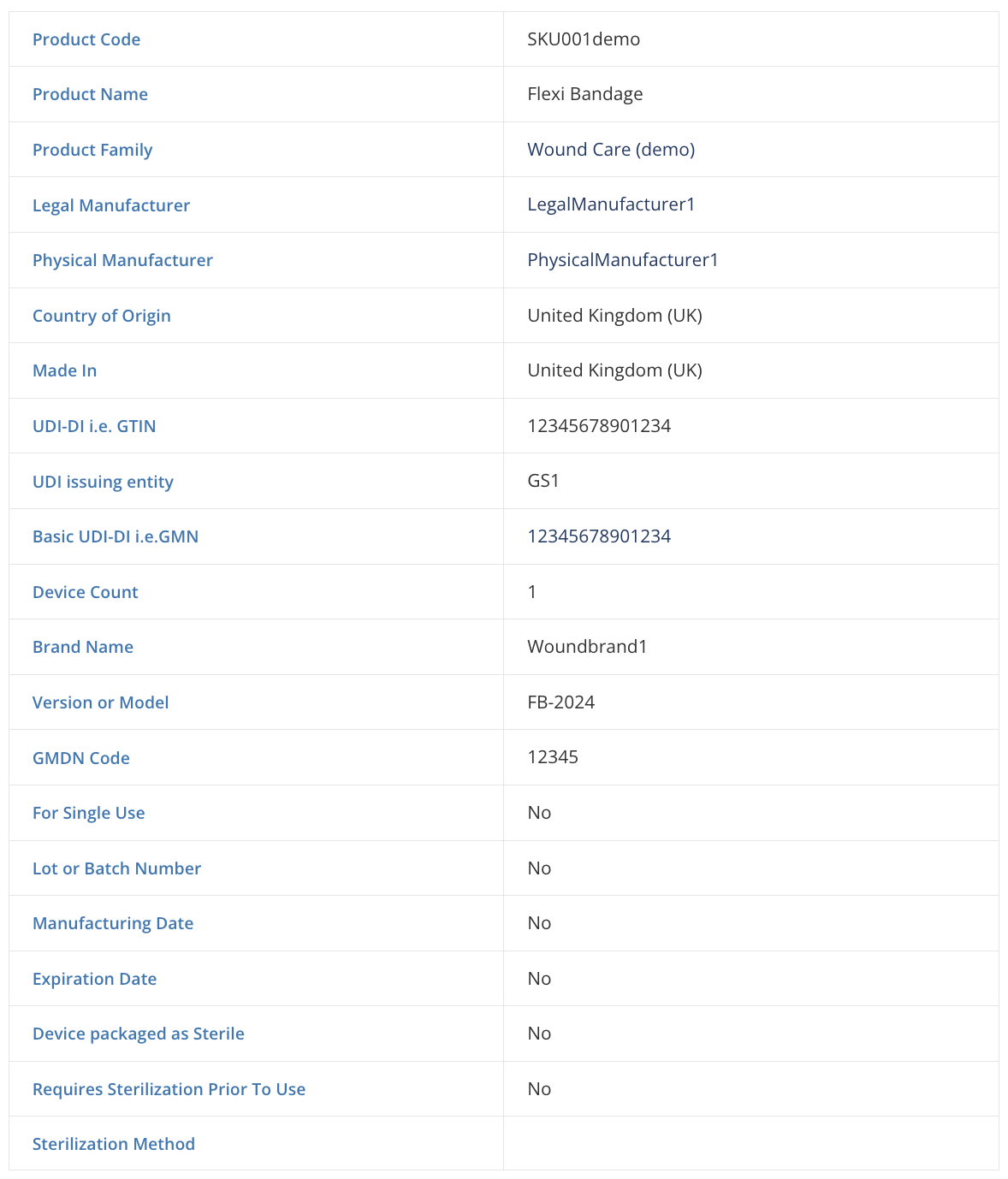
Build any product data model
More than 25 attributes per Product Code/SKU and custom information related to your product. Fields include UDI requirements in USA and EU.
Organize by families and manufacturers
Manage your products automatically grouped by Families, UDIs, legal manufacturers, and physical manufacturers.
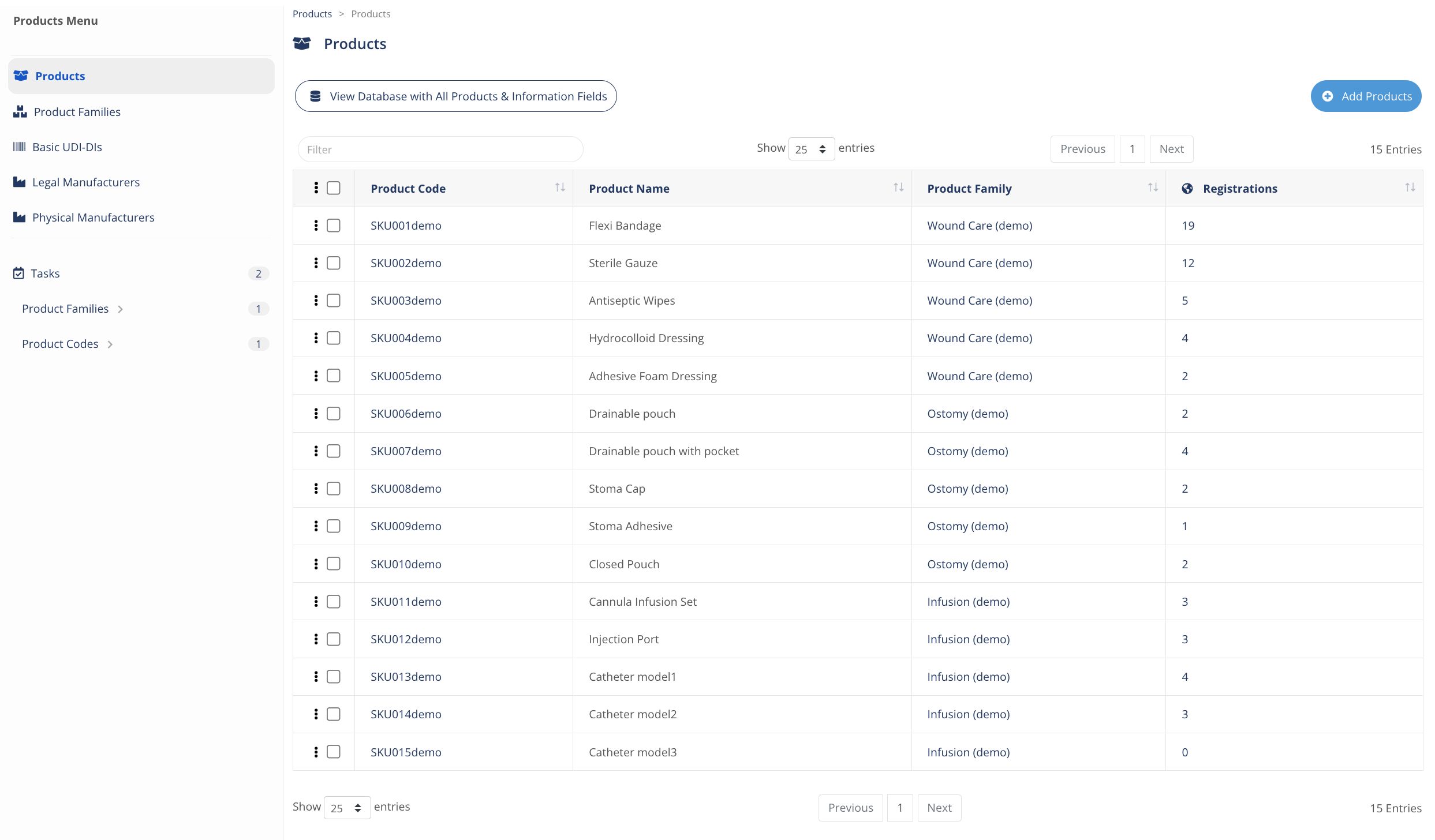
Find and manage products
Embedded with advanced search engine and powerful bulk editing features, access, monitor and filter products information anytime.
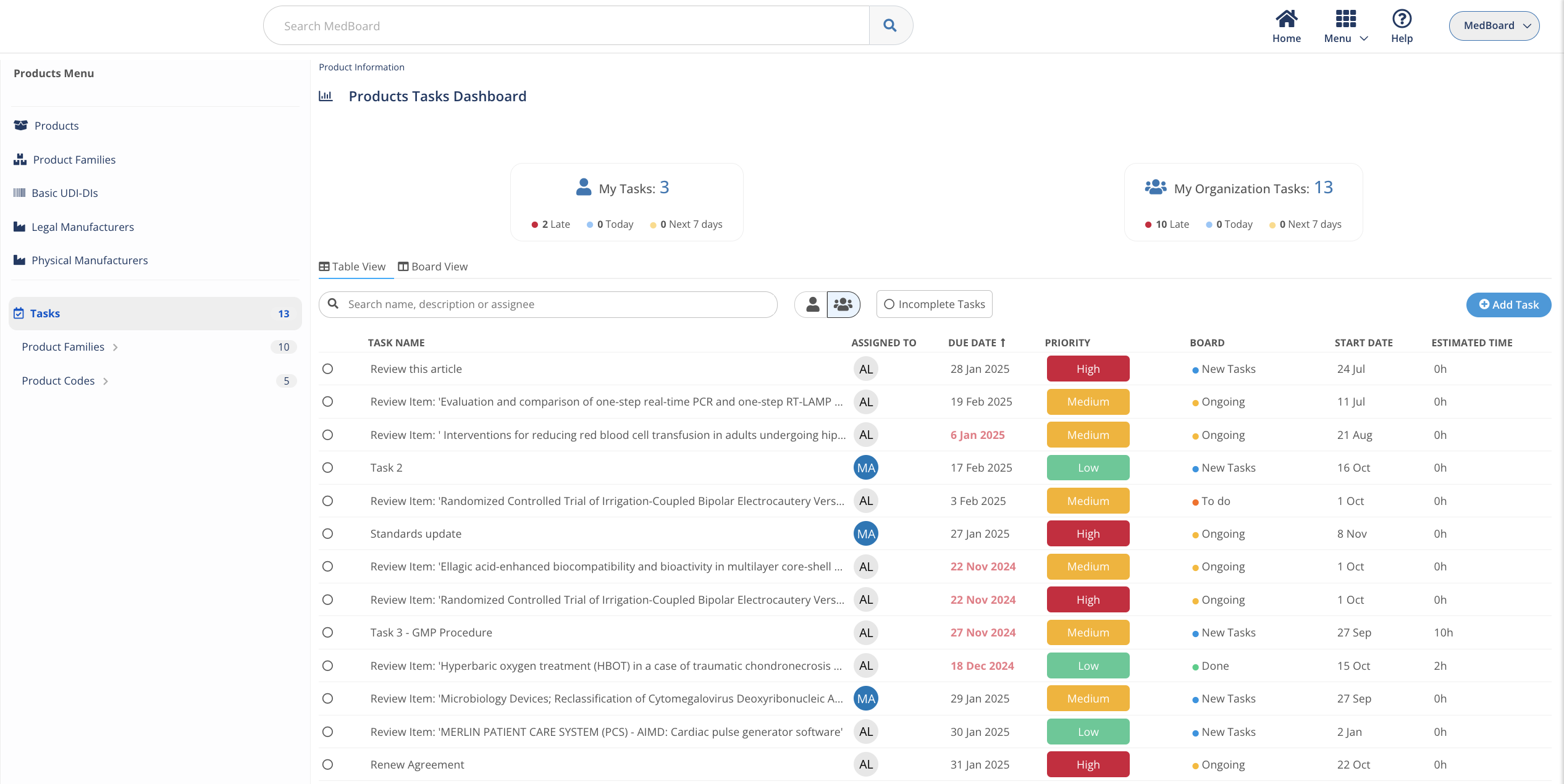
Integrated tasks and automations
An integrated project management solution to keep track of required action, collaborate, and create tasks and deadlines.
Manage access and permissions
Control which user has access to edit and keep your product data 100% safe and secure, supported as well with automated backups.
Export Information and reports
Use the automated dashboard and reporting to get information on the fly, or export data any time in different formats.
Powerful Integrations
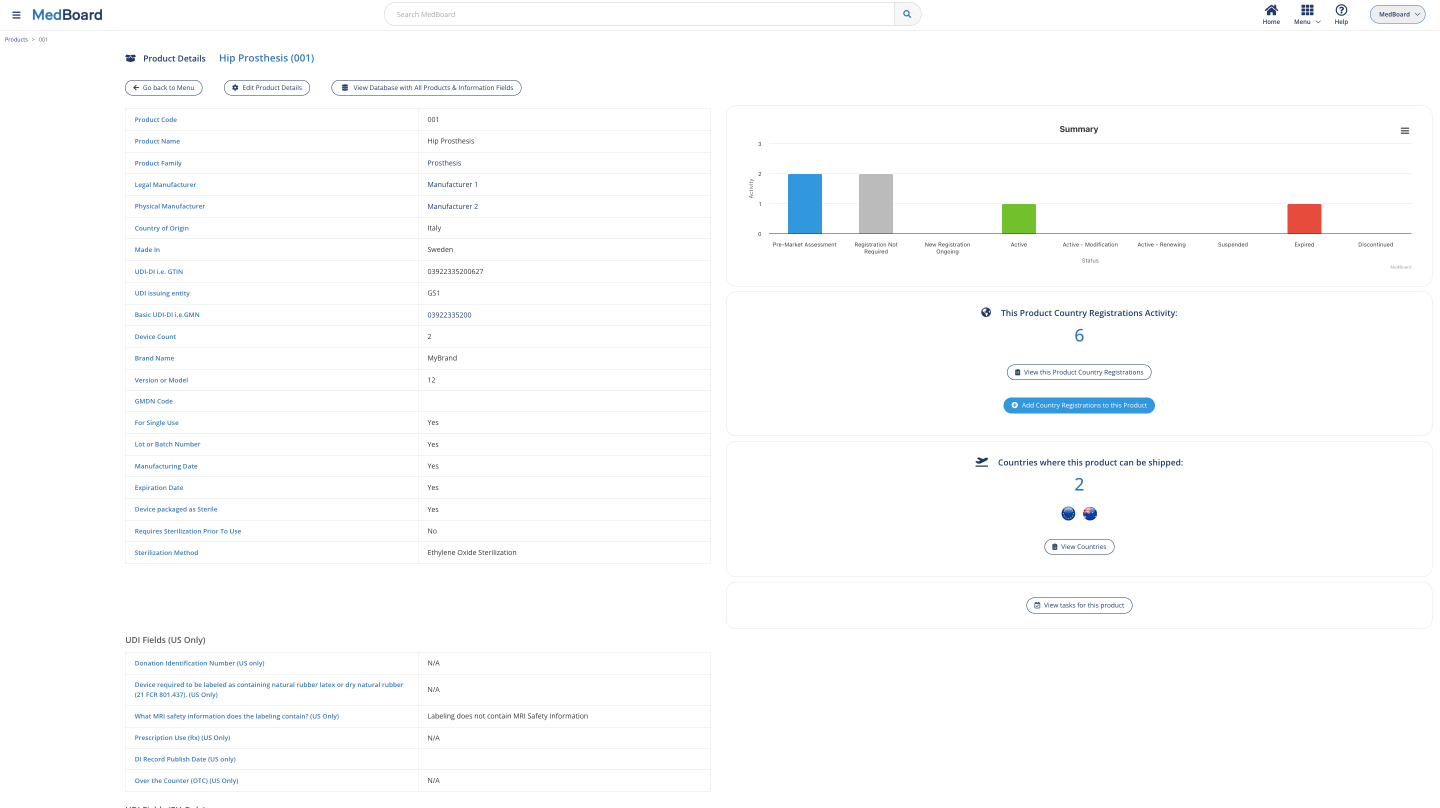
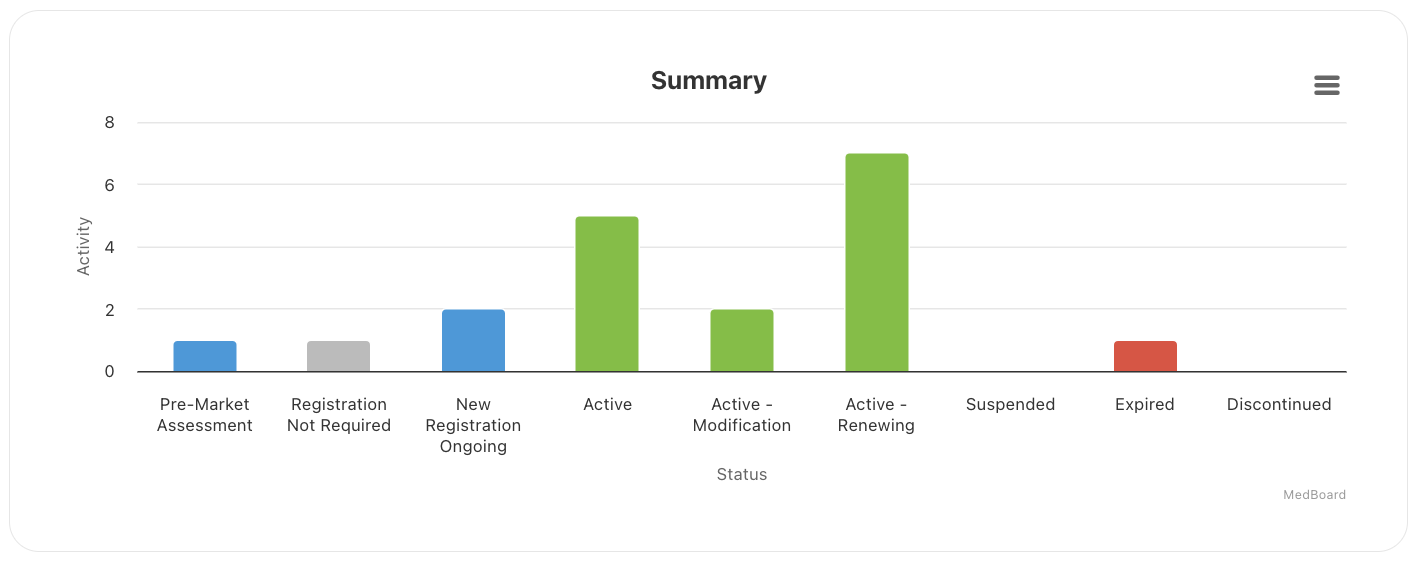
MedBoard Country Registrations
The MedBoard Products Information is seamlessly integrated with the MedBoard Country Registrations RIMS. Visualize Country Registrations and certificates by products, and all their related information and latest news.
MedBoard Compliance Manager
Directly link products with standards, regulatory documents and technical documentation through the Compliance Manager.
MedBoard Regulatory Reviews
Directly link impact assessments and to products and product families for full traceability and implementation.
REST API
MedBoard can also connect with ERP systems and any internal systems.

Make the Most out of MedBoard
Discover our Software Products for Regulatory Needs and Professionals:
![]()
Access to up to date Regulatory Intelligence, data, tools and News in more than 225 Countries, organized, curated and cleaned by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma/BioTech and Clinical trials, that includes countries curated summaries for an unparallel research and intelligence.
Add Documents and Standard that apply to your organization to the Compliance Manager!
![]()
Review systematically Regulatory News and Updates connected to our Global Data covering 225+ countries and 15+ regulatory areas in real time. Customize it, create protocols, impact assessments, actions, and reports easily with MedBoard.
![]()
Organize, manage and track your compliance evidence with the Compliance Manager. This module helps to identify and control the specific regulations, guidance, standards, procedures, technical documentation that apply to you, and to organize, action, and track your related compliance evidence.
![]()
A powerful ready-to-use Products Information Management to organize, manage and track information about your products, product codes, SKUs, and its information, including Unique Identifiers (e.g. UDI), all integrated together with Regulatory Intelligence and MedBoard Search.
![]()
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, licenses and economic operators, all integrated together with Regulatory Intelligence, Regulatory Reviews, Task Manager and MedBoard Search.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams
