White Paper
Navigating Regulatory Complexity
How MedBoard Simplifies Monitoring & Compliance
by Yann Cailler
Executive Summary
The medical device, in vitro diagnostics, and pharmaceutical industries are facing increasing challenges in managing the complexities of a fragmented and evolving regulatory landscape. Regulatory affairs professionals struggle with fragmented information sources, frequent regulatory updates, and resource constraints, making it difficult to maintain compliance and ensure timely product oversight. These issues can lead to costly delays and increased risk of non-compliance, especially as the regulatory environment lacks harmonization and coordination across global markets.
MedBoard addresses these challenges with a centralized platform that integrates regulatory intelligence, workflow automation, and artificial intelligence to streamline compliance tasks. Key features include real-time regulatory updates, advanced search capabilities, a compliance manager dashboard, and a regulatory information management system (RIMS). By automating routine tasks and providing proactive insights, MedBoard helps regulatory teams save time, reduce risk, and focus on strategic initiatives. This comprehensive solution delivers long-term value, ensuring businesses can navigate regulatory complexities while supporting innovation and minimizing compliance risks.
1 Current Landscape of Regulatory Compliance
1.1 Introduction
The medical devices, in vitro diagnostics and the pharmaceutical industry are facing increasing challenges in keeping up with the applicable regulatory environment. Companies and especially regulatory affairs professionals must navigate an ever-changing regulatory landscape in a fragmented world lacking clarity, harmonization and coordination, despite best efforts from regulators.
The increasing complexity is creating a high risk of non-compliance and regulatory affairs are struggling to maintain a balance between ensuring that projects are executed timely, spending enough time to perform regulatory intelligence and spreading knowledge and maintain marketed products in compliance.
The lack of coordination between standardization organization and regulators/compliance bodies is adding an additional layer of complexity for manufacturers as current and future expectations and acknowledge state-of-the-art are unclear and can change at any time. Development costs and timeline can be heavily impacted if there is a sudden change in expected compliance triggering additional testing or redesign.
1.2 Issues and Challenges in Regulatory Intelligence
Managers and members of regulatory affairs departments are facing many challenges:
a. Fragmented Information Sources
Most regulatory teams rely on disparate sources such as spreadsheets with links, manual web searches, email alerts, and agency/specialized website review, … Managers are facing challenges in task allocation as this manual approach is both time-consuming and dull. This process is also generating a lot of distraction for the team members due to multiple and duplicate e-mail alerts and internal e-mail forwarded by colleagues and other departments. Outcomes are dependent on the person(s) assigned to the task and prone to information gap.
b. Keeping Up with Frequent Updates
Due to the volume of the continuous stream of new requirements, especially in companies addressing the global market, tracking of current and future regulatory landscape is a challenge for regulatory teams. Small to mid-sized teams often, due to time constraints, do not properly disseminate the changes in regulatory landscape among the team members and within the organization, making it difficult to achieve a proactive compliance strategy.
c. Impact Assessment and Implementation
Change assessments, which often require subject matter experts from other departments, are sometimes not detailed enough, delayed or simply not performed in a timely manner. The high number of changes open simultaneously at a given time is making it difficult for managers to have an overview and to prioritize resources allocation, often leading to sub-optimum and last-minute implementation. For the same reason, regulatory experts are also struggling to make a pragmatic and holistic implementation instead of sequential updates.
d. Product Maintenance and Update
Being able to maintain a clear overview of which product is impacted by a particular standard update is crucial. For small companies without a dedicated team for product maintenance, ensuring products’ technical documentation is updated timely, in all jurisdictions is a strenuous for the regulatory affairs team.
e. Regulatory approval oversight
Keeping an overview of which device or variant is approved/cleared in which region can quickly become a challenge for regulatory affairs teams. Often knowledge is held by specific individuals who have been working in the same department for a long time and records are buried somewhere in the basement. Failure to maintain overview and timely access to records can lead to recalls, violations, and significant financial and reputational harm.
2. MedBoard: The solution to streamline compliance & resources
MedBoard is more than a simple regulatory intelligence platform, it is an ecosystem of solutions enabling marketing, R&D, clinical, and regulatory affairs professionals to perform better, smarter and faster. MedBoard is specifically targeting the needs of people involved in the development, compliance, and commercialization of medical devices, in-vitro diagnostics and pharmaceuticals. By centralizing market, clinical, and regulatory information and adding workflow. Automation, and artificial intelligence features, MedBoard provides robust data management tools, addressing the industry’s core compliance challenges.
2.1 Key Features of MedBoard
a) Centralized Access to Trusted Information
MedBoard aggregates regulatory updates from numerous regulatory agencies, standardization organizations, scientific repository, and trusted regulatory news websites. New information are continuously added to MedBoard dataset. Never missed an update, thanks to customizable alerts and notifications.
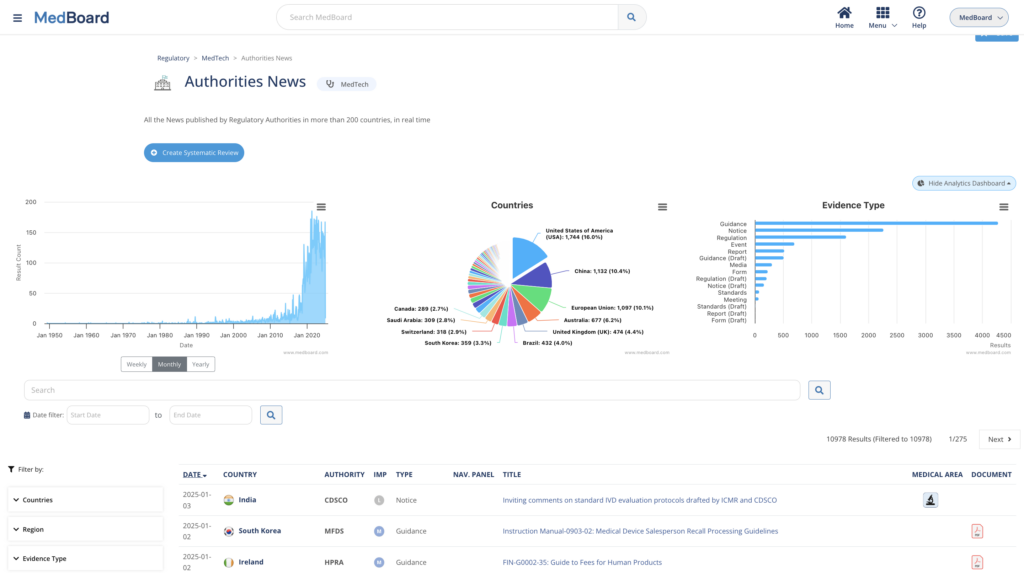
b) Advanced Search Capabilities
MedBoard allows to browse information by category (country, topic, agency, …) or based on pre-defined search criteria. Information can also be consulted through reports, automated collection, and news feeds. Elastic search using Boolean operators can be used to find information throughout the entire database.
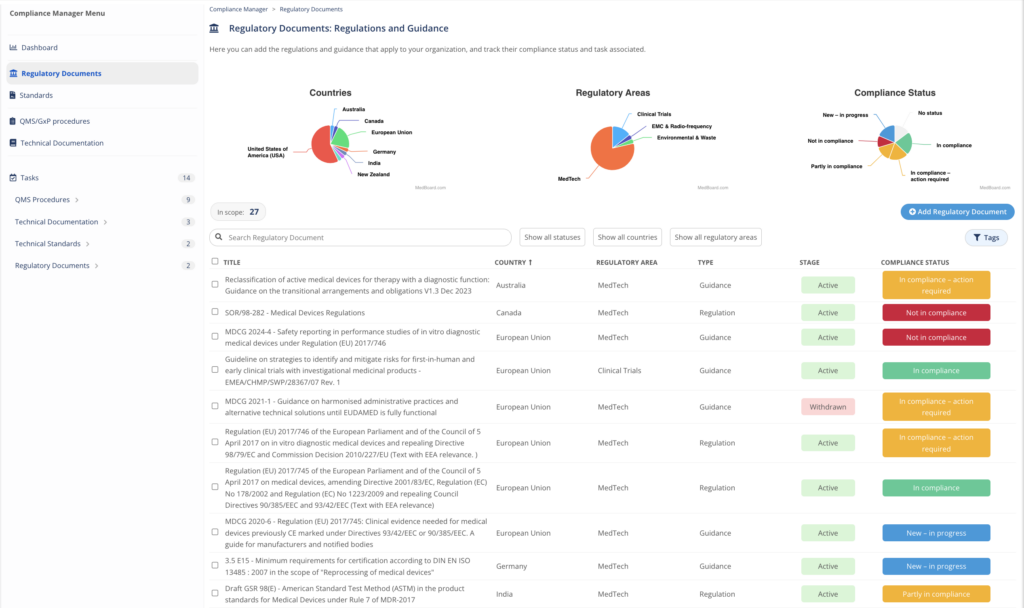
c) Compliance Manager
The compliance manager’s dashboard provides an overview of the compliance status of the company regarding applicable regulatory documents, quality management system (QMS) procedures and technical documentation (TD) in real time. A task manager is allowing the assignment of activities to regulatory contributors, linking trigger (regulatory document) and target (QMS/TD), and following the task status based on due date.
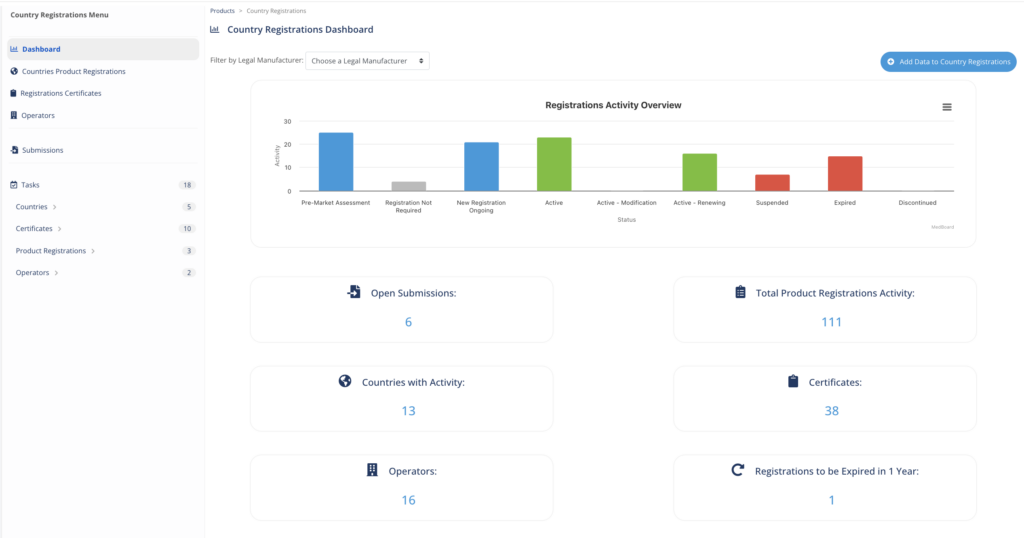
d) Regulatory Information Management System (RIMS)
MedBoard’s regulatory information management system allows to visualize your entire product portfolio and the current registration status at a glance. The product information and country registration module cover all functionalities available in the major RIMS solutions, but it is fully integrated with the regulatory intelligence and the compliance manager functionality, allowing to seamlessly process an item from its publication to a marketed product.
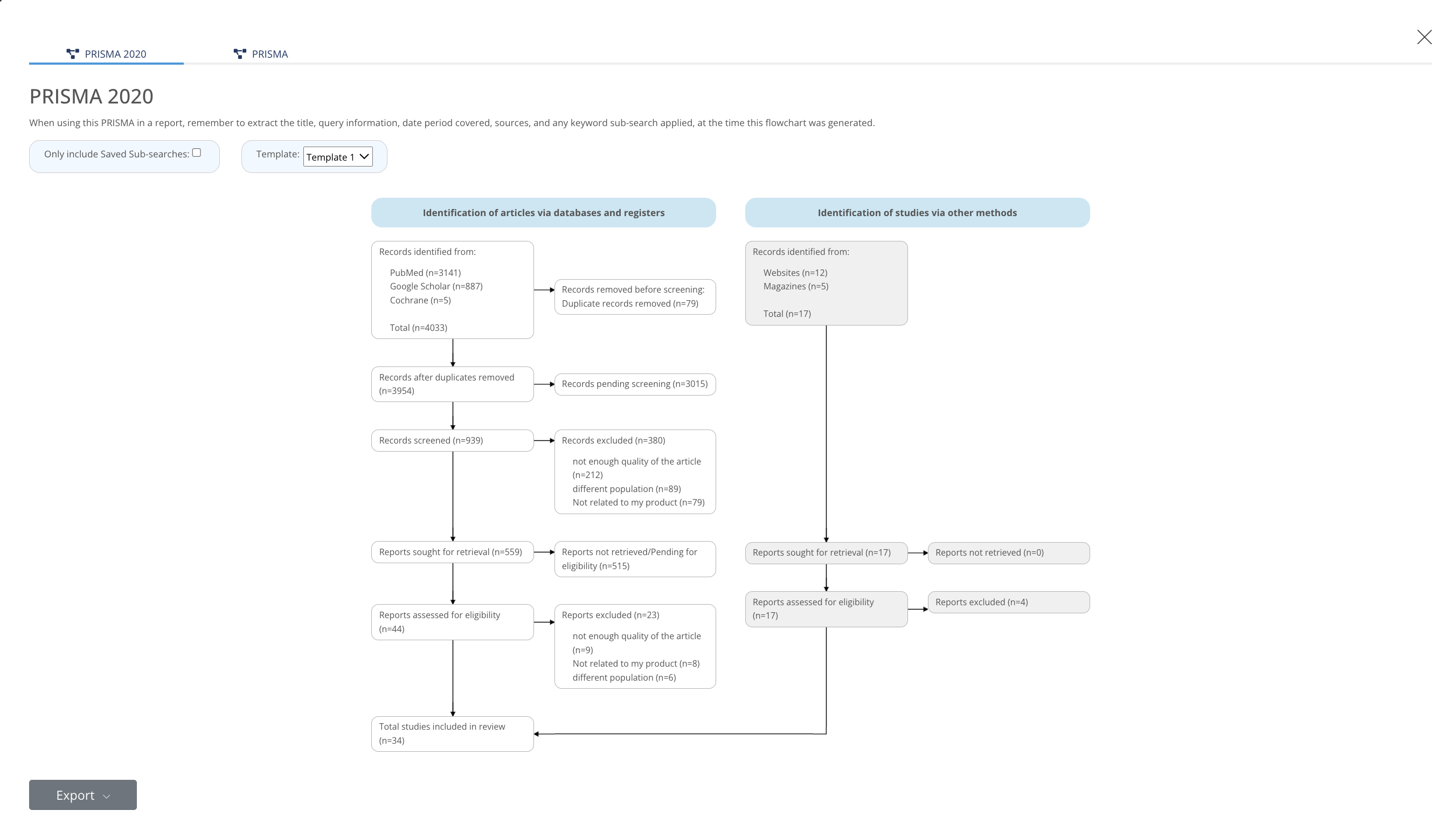
e) Clinical Repository (Literature, Study, Clinical Guideline)
MedBoard’s systematic review (SR) function is removing the need to run queries in multiple websites (PubMed, Google Scholar, …). Articles are automatically imported from external sources and appraisal can be done without using separate excel spreadsheets. Prisma assessments, analytics, and references management are embedded in the platform, removing the need to manually generate information.
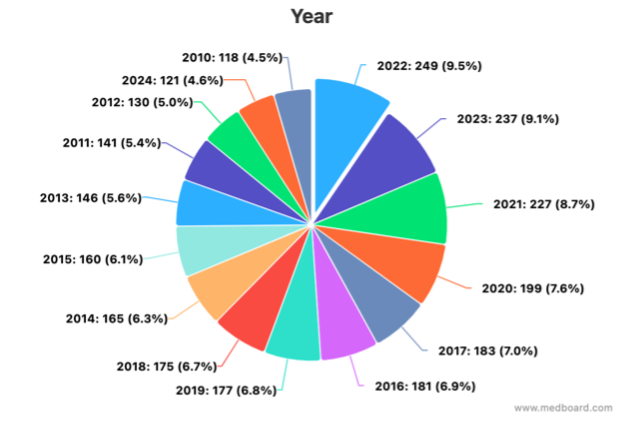
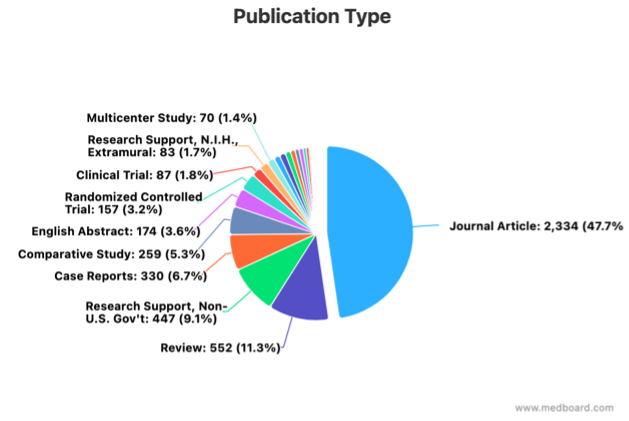
f) Post-Market Surveillance
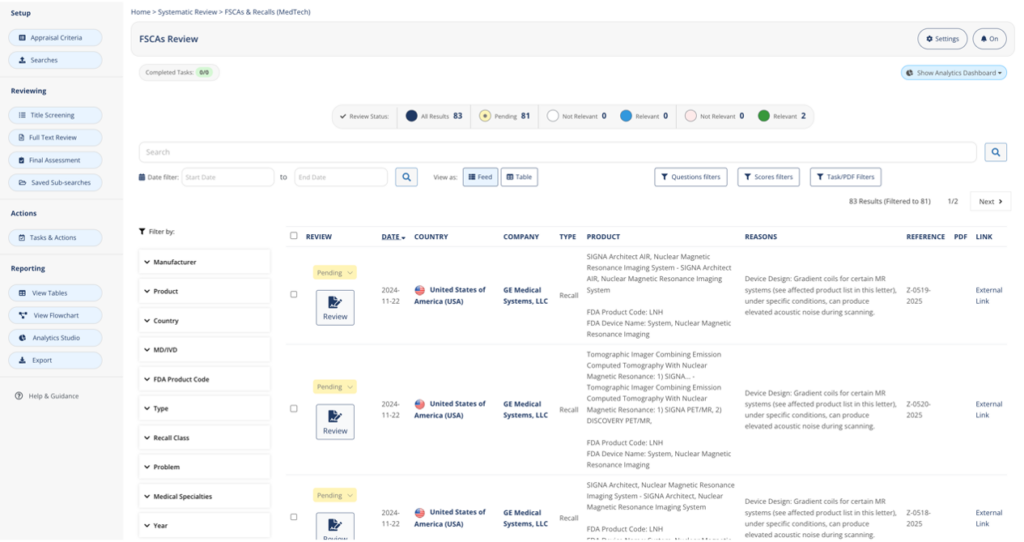
60% of the data needed for creating a PSUR are combined in a single systematic review page. FSCA and recalls are aggregated in a single page with advanced filtering capabilities, removing the need to go through thousands of entries spread among dozens of health agency websites. Cybersecurity alerts and third-party software including libraries and drivers are also readily available with tags, saving a significant amount of time.
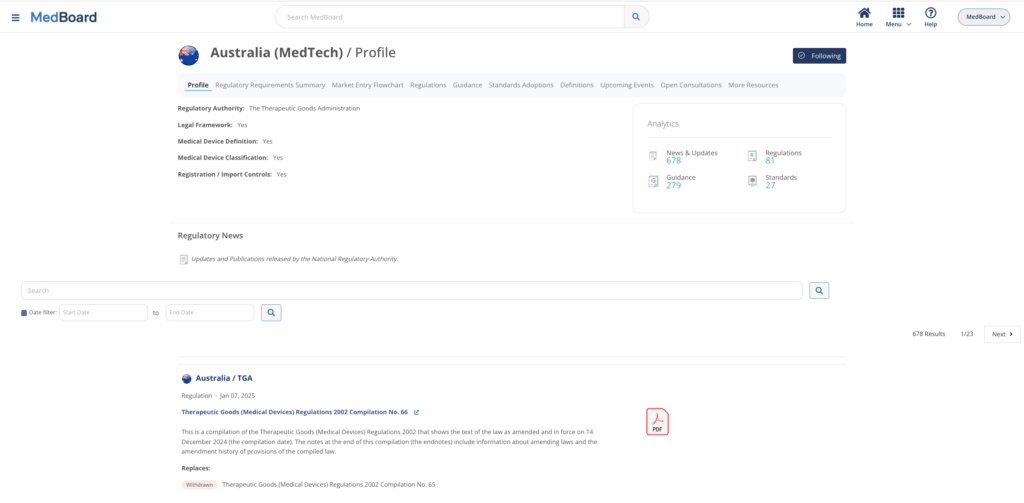
g) Data Visualization and Reporting
With more than a million curated data, MedBoard provides everything you need. Manufacturer profiles (30’000+), hospital & clinics (10’000+), approved devices, inspections records, standards and guidance and much more. A number of tools are available to enable fast identification, such as technical standard lifecycle, standard adoption, gap analysis, essential performance/principles, 510(k) visualization tree, country regulatory requirements, and monthly reports. Numerous analytics dashboards allow visualization of data and trend identification. Another key feature of MedBoard is that all data are interconnected (e.g. Manufacturer -> Product -> Approval -> Clinical Trials or Standard -> Authorities -> Lifecycle -> Regional Adoption).
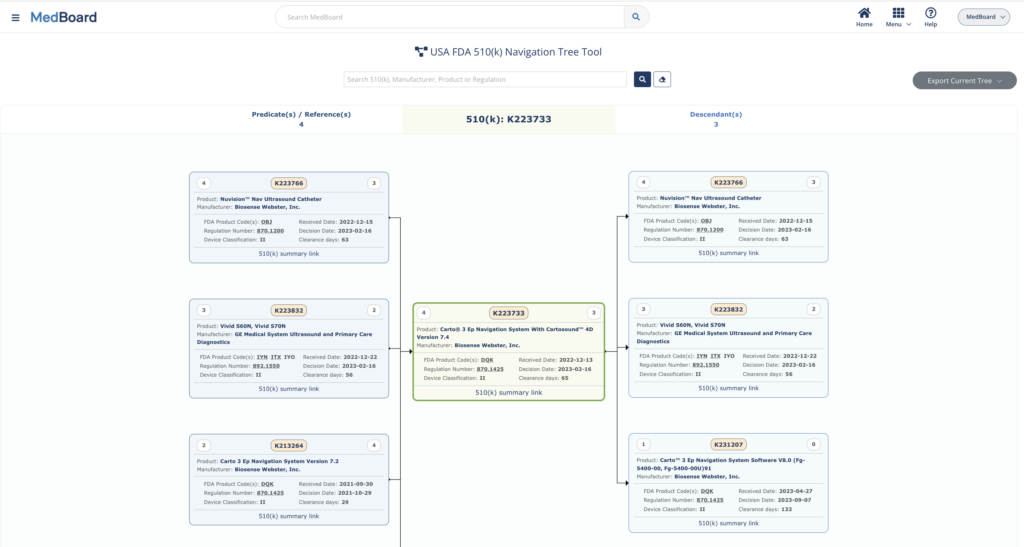
2.2 How MedBoard Addresses Key Regulatory Challenges
Resources constraint is the root cause for most of the challenges regulatory affairs professionals are facing nowadays. The key features of MedBoard enable regulatory teams to decrease the time dedicated to regulatory intelligence and thus allowing to focus resources and energy on demanding and strategic tasks
a) Centralized Information Hub
No more distracting newsletter, broken links or wasting time reading regulatory news only to realize that it is not providing any answer and is a mere marketing tool. With MedBoard as a centralized information hub, regulatory intelligence becomes more stimulating. With the country profile feature, it is possible to quickly get an overview of the requirements for a new target country. Having a single platform improves efficiency, increases accuracy, and reduces the chance of overlooking critical updates.
b) Real-Time Regulatory Updates
Systematic reviews can be customized to each company’s needs through queries with Boolean operators. Systematic reviews can be assigned to specific individuals, be time bound and be continuous. Once the needed systematic reviews parameters have been set, MedBoard will automatically add new relevant information in real time. There is the option to be notified by e-mail when new information are added. New information are set with a pending review status in order to easily identify new items. This feature enables proactive monitoring while minimizing manual tasks, thus allowing professionals to be focused on their current priority tasks.
c) Review & Task Management
Creating separate excel spreadsheet or word document and sharing them via e-mails if a practice from the past. New information review can be assigned to a specific individual with associated due date and the review can be performed within MedBoard platform. Tasks attributes such as priority, owner, timeliness, time needed and dependencies to market, product, can be visualized through a dashboard, allowing managers to have an overview of the tasks and resources allocation, leading to better control and optimized implementation. Regulatory experts can plan pragmatic and holistic implementation by seeing dependencies to any other regulatory information regulatory, technical, and QMS documents.
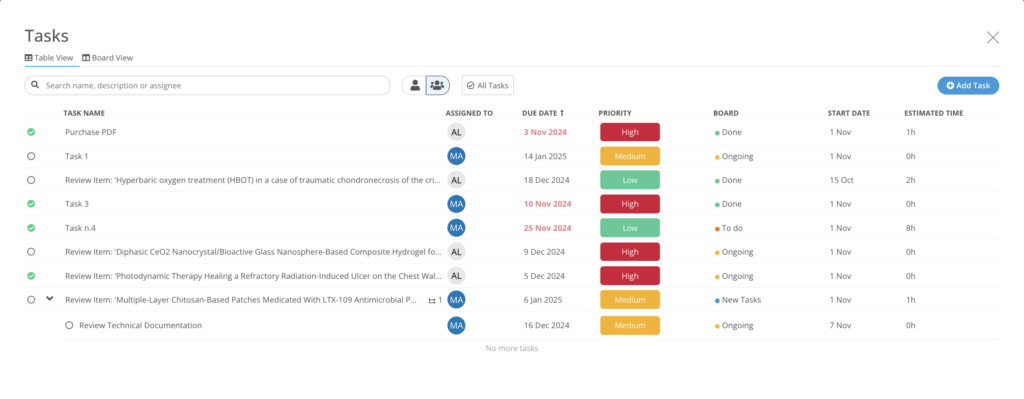
d) Compliance Overview
The compliance manager module provides a complete overview of your compliance status. The integration of the compliance manager, tasks manager, and the database enable full traceability by keeping information connected.
e) Regulatory Information Management System
MedBoard allows tracking and oversight of product registration, product’s related information such as UDIs, regulatory correspondents (importers, distributors, representatives) as well as storage and sharing of regulatory documentations (certificates, FSC, DOC, …). Manage expiration of product registrations, internal and external certificates, selling status, and associated tasks to keep business risk of releasing non-compliant products as low as possible.
3. Future outlook
The regulatory landscape is expected to continue to evolve rapidly in a non-harmonized way and resources constraints in regulatory affairs departments is not expected to disappear. The wave of information and resource constraint is exacerbated by manual, non-coordinated and non-centralized regulatory intelligence, clinical data and product oversight tasks MedBoard’s commitment to real-time intelligence positions it as an invaluable partner for quality and regulatory affairs teams. As regulations become more complex, platforms like MedBoard will play an essential role in supporting compliance strategies and enabling organizations to navigate the future of regulatory requirements.
A proactive approach to compliance allows medical device companies to focus on innovation while minimizing regulatory risks. MedBoard equips teams with timely insights, reducing time spent on routine monitoring and enabling them to address regulatory changes before they impact operations.
4. MedBoard Value and ROI
To make it simple, if by using MedBoard you save 5 man-days over a year, your return on investment is guaranteed. MedBoard agility and ability to cover regulatory intelligence, regulatory information management system and clinical data management is removing the need to manage multiple software subscriptions, going through lengthy deployment process, and reducing the number of trainings to be managed for new team members.
MedBoard is not only leading to time-saving by improving efficiency, allowing to accomplish more with fewer resources and removing the stain of resources constraints. The platform will deliver long-term value, as the time saved on routine tasks allows teams to focus on key strategic initiatives, strengthening the return on investment and reducing the risk of compliance lapses leading to costly penalties and unvaluable reputation damages.
According to the 2025 MedBoard Customer Survey, covering 350 users, from large organizations to start-ups, 86% of users reported an average of 50% reduction in manual work and 76% of users reported that MedBoard is allowing them to process regulatory intelligence 3 to 10 times faster. More than 80% of users have noticed that using MedBoard is leading to improved quality and consistency.
In addition to better performances, the survey highlighted that 87% of users report that MedBoard has positively impacted their job satisfaction, in many ways from feeling less overwhelmed to a decrease in procrastination.
5. Conclusion
MedBoard is transforming the way quality and regulatory affairs professionals manage overall compliance of its documentation, product and clinical data in the medical device in-vitro diagnostic and pharma industry. By bringing automation and big data into a single fully integrated system, MedBoard empowers both managers and contributors of regulatory teams to stay ahead of regulatory changes, mitigate compliance risks, and focus on innovation.
Next regulatory is at your disposal to explore how MedBoard can benefit your organization, discuss your specific needs or request a demo. We can also assist you in the deployment and customization of the platform to your individual needs.
Visit our website www.nextregulatory.com or get in touch at info@nextregulatory.com!
About the Author

Yann Cailler
Yann has a BSc in Microengineering and an MSc in Health and Pharmaco-economics.
With 10 years’ experience in quality and regulatory in the consultancy, industry and certification sector both as a contributor and as a manager, Yann is a seasoned regulatory affairs professional. Yann is specialized in cybersecurity, artificial intelligence, and global product commercialization for active, implantable, and combination products.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams

