Customer Article – The Other Consultants

Adam Isascs Rae is a medical device quality and regulatory consultant. With a 10 year experience, he is a trusted partner in delivering pragmatic and risk-based advice to enable organisations place safe and effective devices on the market, while considering their business goals.
He provides work as a medical device consultant, but he is also a qualified lead auditor who works with certification bodies. This gives him a unique view to understand the perspective of both sides of the table – the auditor and the auditee.
Adam, as a regulatory professional, shared his perspective on the use of MedBoard in his work.
How I use MedBoard as a Regulatory Consultant
by Adam Isaacs Rae
Now, more than ever, quality and regulatory professionals in the life sciences industry must keep abreast of current going ons.
Now we have the UK MDR, EU MDR, EU IVDR, ISO 13485, QSR (which will become 13485), MDSAP, ISO 10993, ANIVSA requirements, MDCG, IMDRF…Do I need to keep going to prove my point? There. Are. LOADS.
Most Medical Device manufacturers will be familiar with the requirement to manage Documents of External Origin (DXOs) to keep up to date with any changes. Not only is this an ISO 13485 requirement, it is a requirement for any organisation that has used a guidance document as a way to implement elements of regulatory requirements into their Quality Management System (QMS).
Life Before MedBoard
Before using MedBoard, I had a list of locations and contacts I would follow to keep on top of updates.
This would be a combination of some of our favourite creators on LinkedIn, European Commission website, MDCG guidance document tracker, ISO website and government websites. This is really not an exhaustive list but is all that comes to mind for now. Although there are many more. I created a simple system for this, however, whilst I am confident enough to call it a system – it was rudimentary at best.
Further, one risk with my “systemˮ was that I would sometimes miss information. Another secondary risk was that I would often see the same information several times, which is interesting to see different perspectives, but is also a waste of time and energy.
You know that feeling, when a new MDCG guidance document is released and EVERYONE is talking about the same thing. Yes, thatʼs exactly what Iʼm referring to.
I find it slightly impossible to estimate how much time I spent administrating this system, as it would run throughout the week extremely sporadically.
Life with MedBoard
The single biggest difference MedBoard has made is having a singular location for everything I need to look at.
I now spend around two hours per week to review everything that has been pulled into MedBoard and schedule it into my reading pile.I no longer need to spend my time going from website to website actively looking for things.
My process is now, simply – login to MedBoard…check what has happened in the last week, use the links and shortlist what I am interested in reading. As shown below, I can just click here and it takes me straight to the location of the source document.
On top of general updates to things going on, having access to the vast amount of literature available through the platform is really powerful.
MedBoard's Impact Assessment Features - Systematic Reviews
Regulatory News Systematic Reviews
One of the key functionalities MedBoard provides is the ability to perform detailed impact assessments with ease. As a regulatory consultant, itʼs essential to understand how each new update will affect the various regulatory requirements I manage for my clients. MedBoard simplifies this process by enabling me to categorize and map new regulatory changes to specific product lines, ensuring that I can quickly assess which parts of a companyʼs processes need adjustment.
The platform also allows me to quickly track whether a regulatory update has been addressed in the Quality Management System (QMS). This helps identify which documents need to be reviewed or revised without combing through entire regulatory documents manually.
We are at the end of the year now, which provides us a nice time to evaluate all of the documents from the year. Below is an analytics screenshot of the year from 1st January 2024 until 21st December 2024.
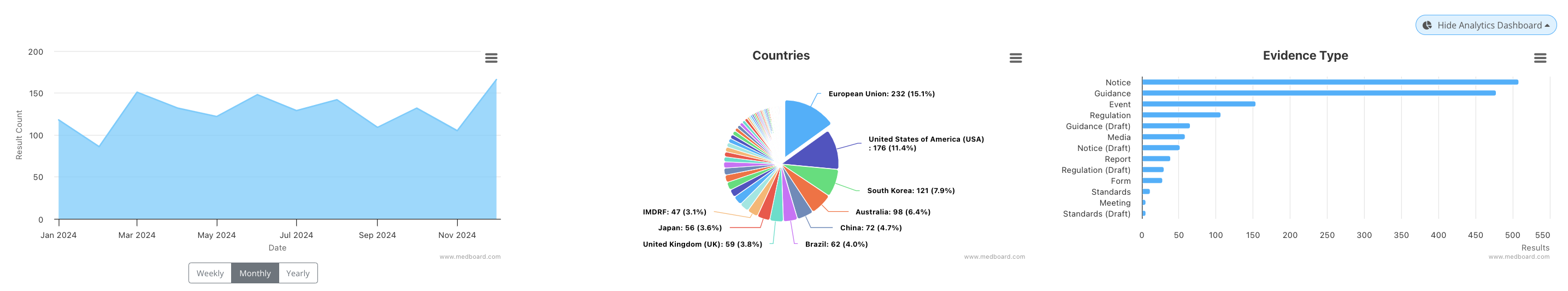
Firstly, MedBoard is showing here that we can look at the global regulatory framework. If we look on the graph on the far right, we can see that just this year, there have been on the upper end of 400 notices and guidance documents released globally.
For a one man band (yours truly) or global consulting firm, that is a lot of work to keep up with.
Another valuable feature MedBoard offers is its support for systematic reviews. When regulatory changes are released, I need to perform a systematic review to evaluate how these changes impact multiple areas of compliance. MedBoard’s categorization system allows me to tag relevant regulations, standards, and guidance documents to make sure I stay organized and do not overlook any vital details.
Moreover, MedBoard automatically gathers updates from key sources and centralizes them in one place, which makes conducting a systematic review much more efficient. Instead of manually collecting documents and analyzing their relevance, MedBoard provides a streamlined process that allows me to see at a glance whether a document affects existing regulatory frameworks.
For example, when a new ISO 13485 standard is released, MedBoard will highlight the update and link it directly to the products and processes that could be impacted. This level of automation is incredibly helpful for making timely, well-informed decisions about the necessary changes to my clientsʼ compliance strategies.
With MedBoardʼs systematic review features, I can ensure that all regulatory changes are accurately assessed and incorporated into my clients’ QMS, with minimal risk of missing important updates or misinterpreting regulatory changes
Market and Post-Market Surveillance (PMS) Systematic Reviews
Standards and regulations have talked about a risk based approach for many years now, however, more than ever we need to be able to use data in order to manage risk.
A key question that every regulatory professional must ask themselves, and to their peers, colleagues and clients is:
What is the risk?
The answer to this can never be simply, well there is none. That doesnʼt happen, or exist. Unfortunately for some, but fortunately for the patientʼs often.
Much of my work is managing remediation, i.e., fixing things that maybe werenʼt perfect in the first place, or additionally developing and improving things to match modern regulatory requirements or standards. This means we often need to take a certain approach to dealing with and managing legacy issues. So we often find ourselves asking the question:
What is the risk?
Again, we cannot simply say there is none. If there has been a device on the market, then fortunately we are in a position to be able to leverage some data from PMS. MedBoard makes the process of gathering PMS information far easier by enabling us to conduct swift searches across a number of markets, regulators, or specific device criteria for example.
The systematic review functionality of MedBoard makes this easy to do in a structured, organised and slightly less overwhelming way.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams

