Country Registrations / RIMS
Manage, organize and access global country registrations and operations, with this scalable, customizable, and secure solution.
Purposefully built for the life sciences organizations, manage your registrations,
certificates, submissions, operators and many more very easily in one single place.
All integrated with your Regulatory Intelligence.
Integrated
Seamlessly integrated with MedBoard Products Information and other MedBoard regulatory and clinical solutions.
Scalable
Flexible and scalable system to manage multiple registrations and complex data.
Secure
MedBoard is ISO 27001 certified, ensuring robust information security management practices, and backups.
Have a full lifecycle support over all your registrations registrations
Powerful features and automations
for Country Registrations Management
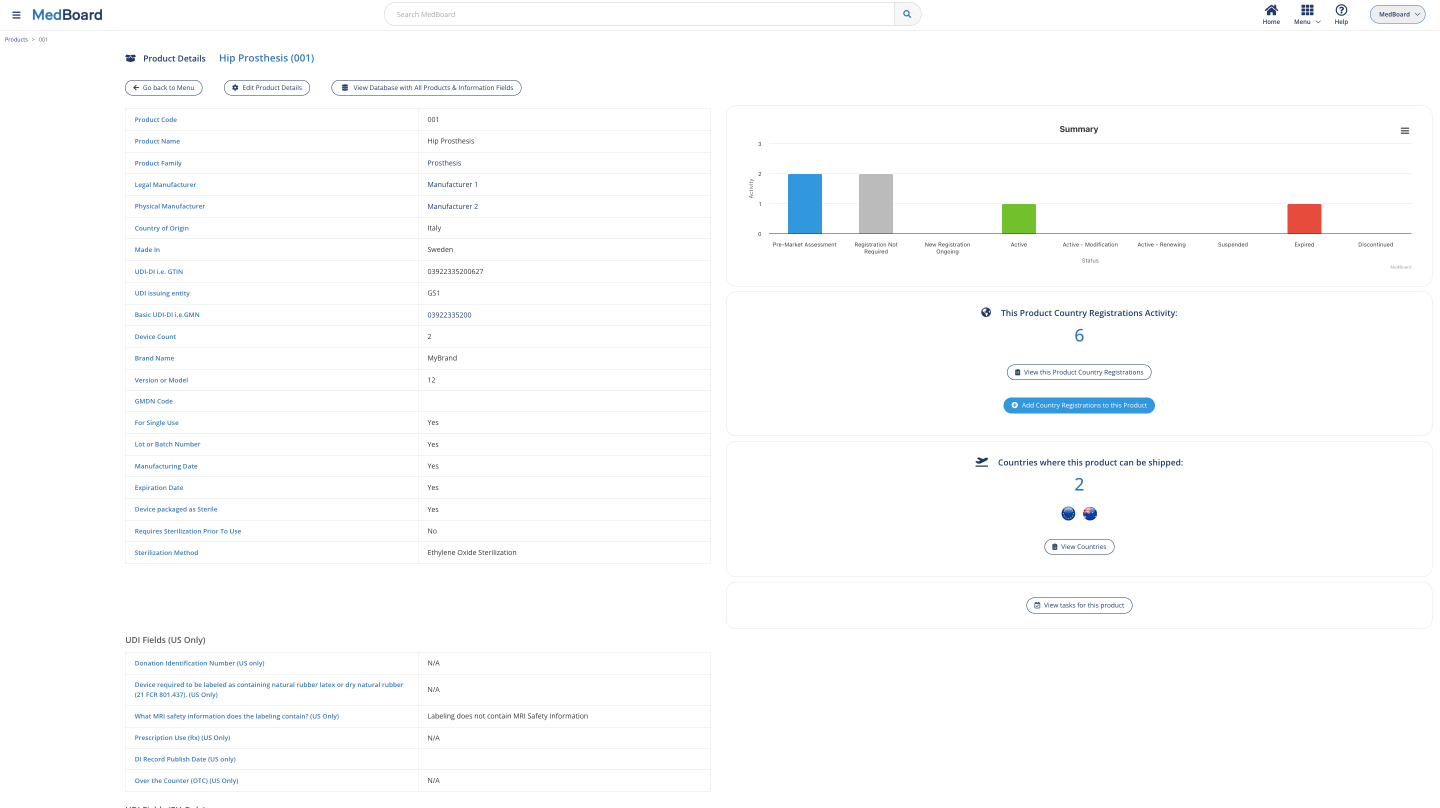
Manage registrations data
Country Registrations
Access to all your Country Registrations data, seamlessly connected to your products, certificates and operators. Visualize all registrations, by country, region, certificate, or manufacturer.
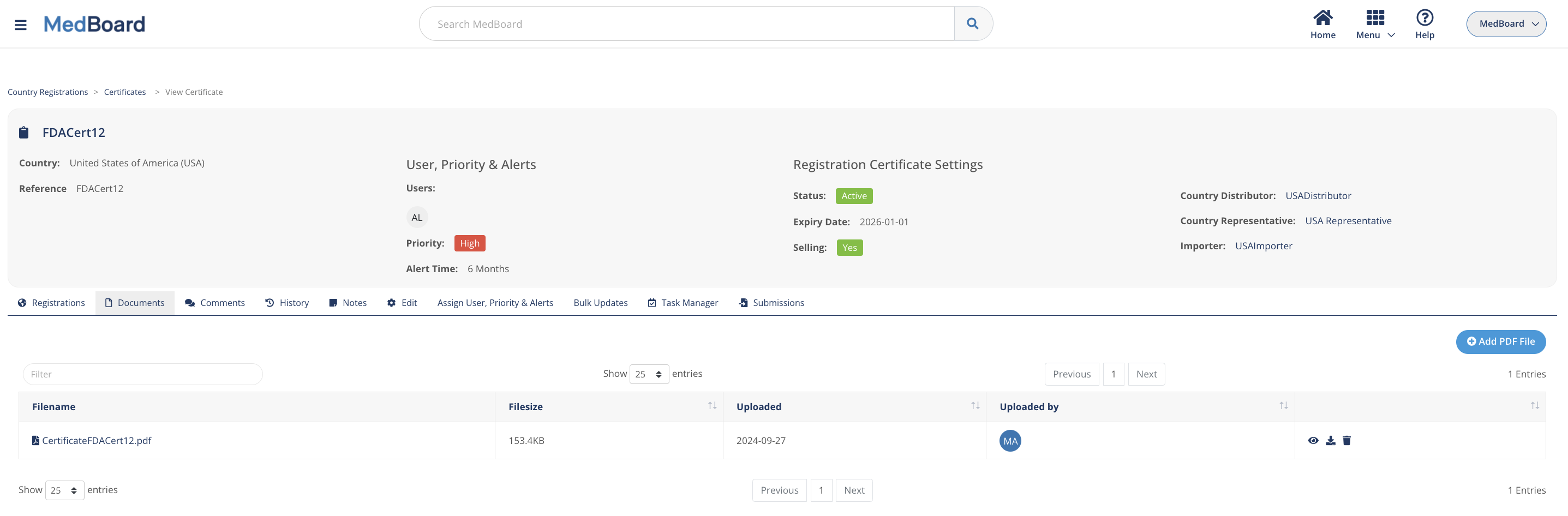
Certificates and licences
Keep control over all your certificates and licenses by country, including any condition or expiration.
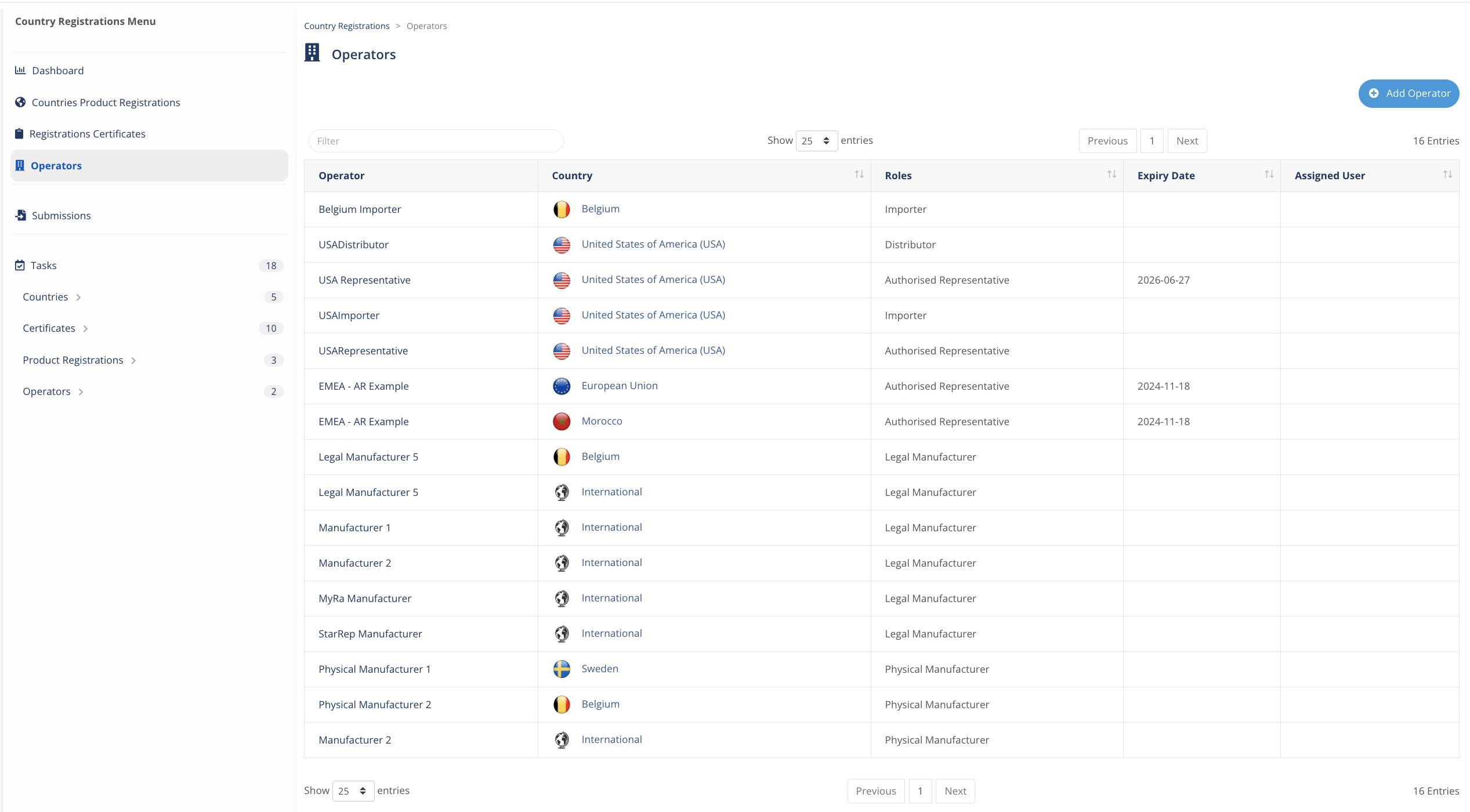
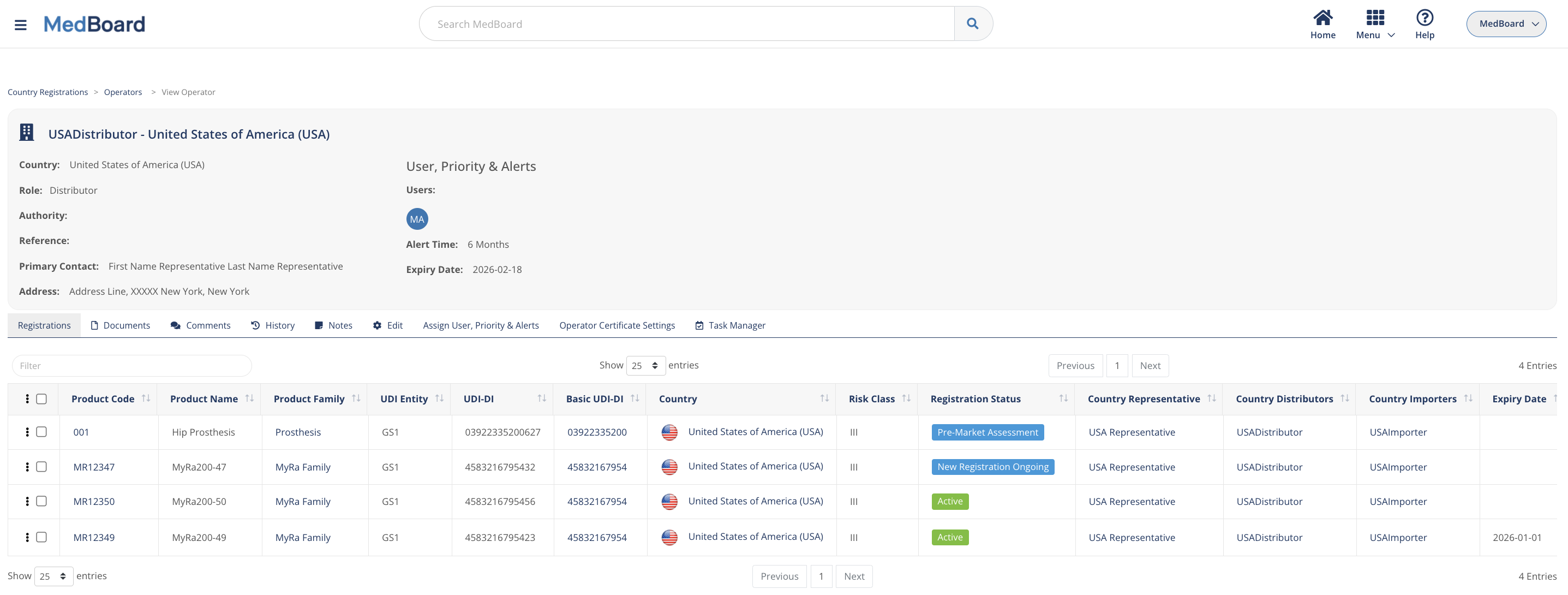
Economic operators
Manage all your Authorized Representatives, Distributors, Importers, Legal and Physical Manufacturers and many more operators.
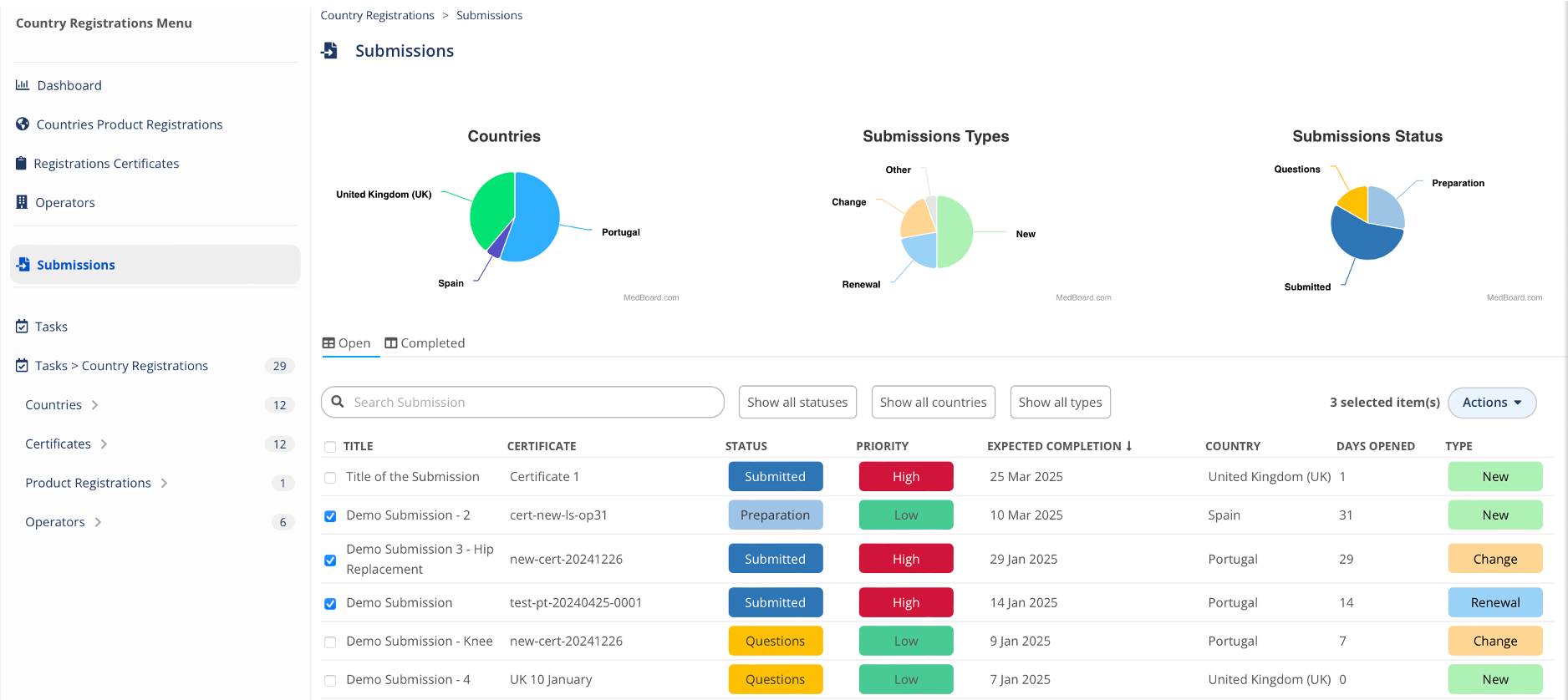
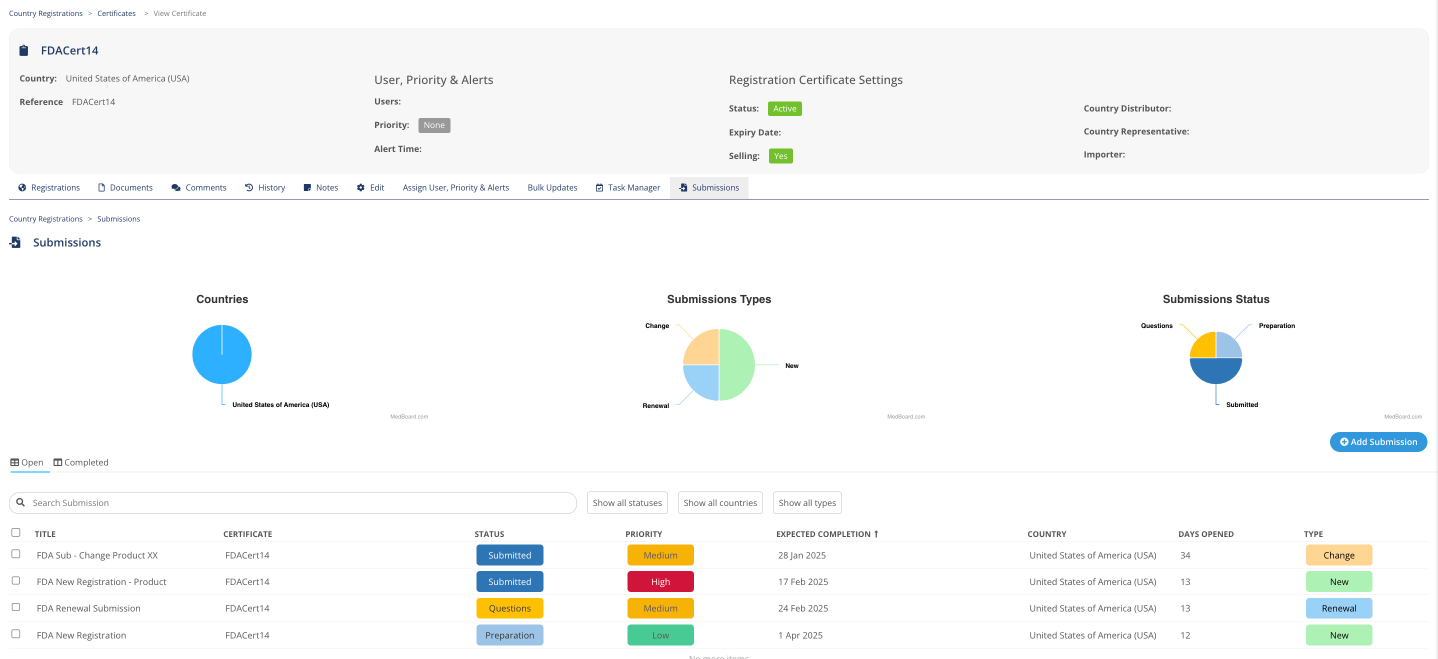
Submissions
Track progress on Submissions at different stages, linked to certificates, expected completion date, responsible, and many more KPIs.
Documents
Upload all the documents related to your registrations and certificates for easy access and reporting.
Regulatory Flags
Control with admin rights which countries and regions products can be shipped, and whether selling is active or not from regulatory perspective.
Manage projects, changes and renewals
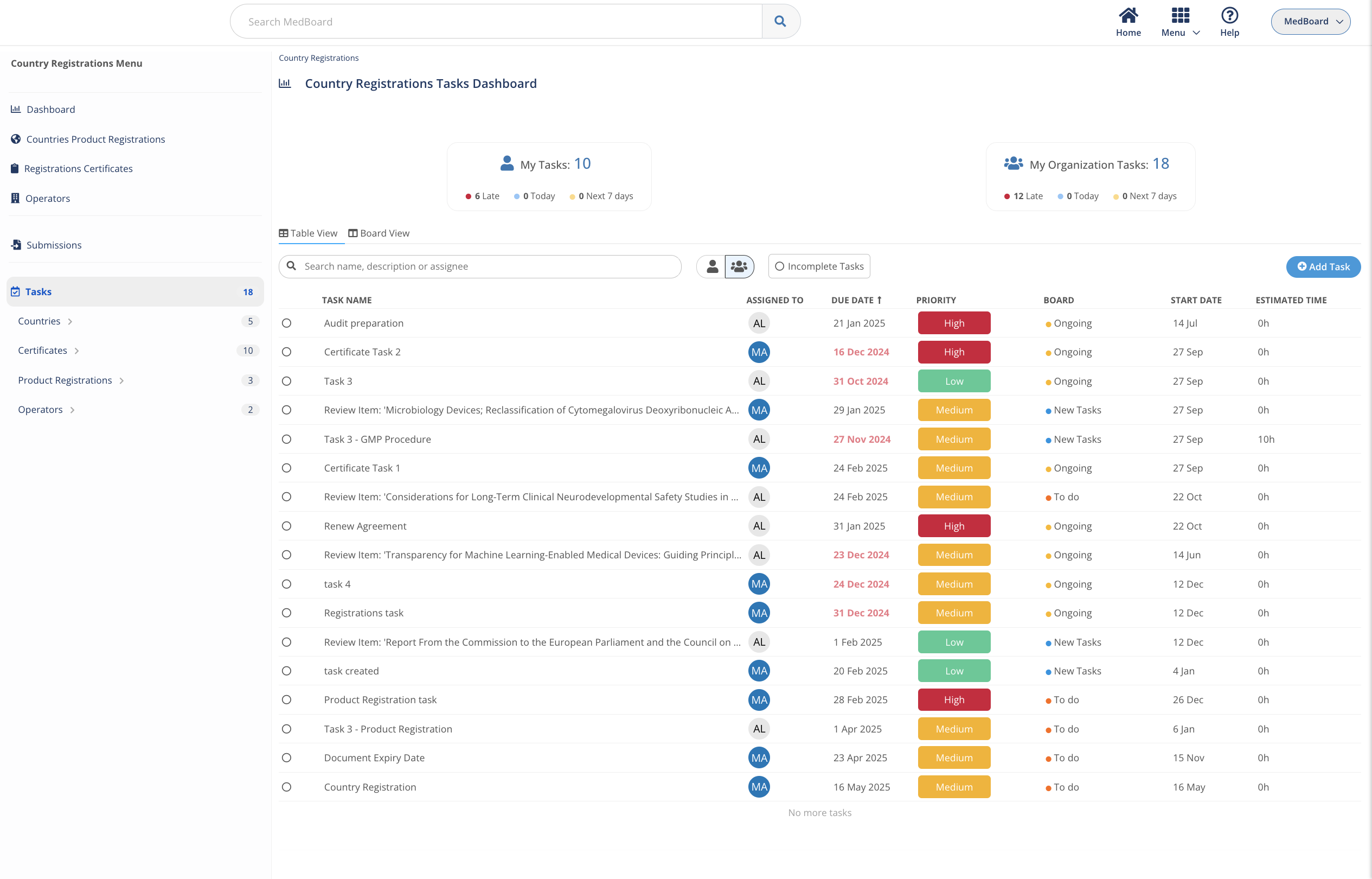
Create actions and tasks
Create actions and tasks linked to your new registration projects, renewals or changes, and have full visibility who and when needs to complete it.
Track deadlines and priorities
Set priorities and deadlines for any important project, whether it is a new registration, a submission or a change, you can have full control on what’s going on so you act quick.
Be on top of submissions
When there are many submissions going on it is difficult to see all the expected completion dates, pending actions and what to prioritize, MedBoard allows you to easily tracking all of them by region and certificate.
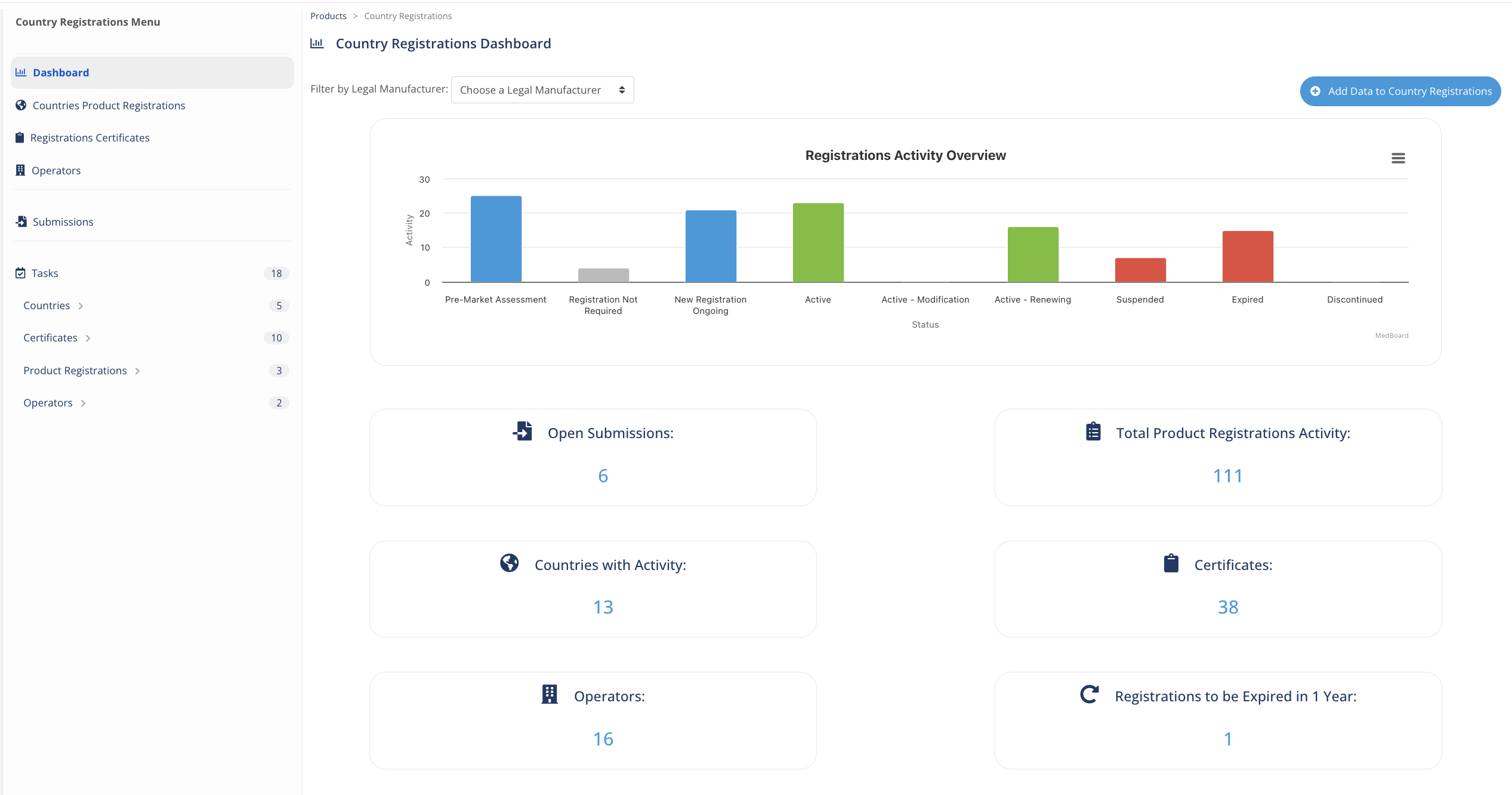
Dashboard tracking
KPIs and risks
Monitor easily key information for you and organization on your registration progress and risks with the custom MedBoard dashboards. For example, you can ask, how many submissions were completed last year?
Expirations
Keep track of all the expirations of registrations, certificates or agreements with notifications, tasks and actions. Assign priority, alert time and responsible user.
Renewals
Monitor renewals on a global scale and plan ahead of time to avoid any costly disruption, whether these are certificates or establishments licenses.
Report and export
Market lifecycle status by product
View information by product on all their registrations activities over all the countries, certificates and operators connected, instantly, with our easy to use dashboards.
Report by different actors and metrics
Export information filtered by manufacturer, certificate, country and many more metrics, with easy to use export functions, ready to use for your reports or audits.
Commercialization status
Report instantly whether a product is commercialised in a specific country and what is the latest status.
MedBoard Integrations
MedBoard Products Information
The MedBoard Products Information module is seamlessly integrated with the Country Registrations RIMS for a fully integrated data experience.
MedBoard Regulatory Intelligence
Use the MedBoard Regulatory Intelligence to research requirements and plan your registrations in any country of the world.
MedBoard Task Manager
Use our integrated Project Management solution to keep track of all the actions, priorities, responsibles, and deadlines, so you do not miss a thing.
100%
CSAT
Customer Satisfaction (CSAT) Score,
with an average overall satisfaction with MedBoard of 4,8/5.
50%
Reduced Manual Work
86% of MedBoard users report reducing
by more than 50% the need for
manual actions and work.
3x-10x
Faster Processes
78% of MedBoard users reported
performing actions 3x to 10x faster,
accelerating their processes.
Make the Most out of MedBoard
Discover our Software Products for Regulatory Needs and Professionals:
![]()
Access to up to date Regulatory Intelligence, data, tools and News in more than 225 Countries, organized, curated and cleaned by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma/BioTech and Clinical trials, that includes countries curated summaries for an unparallel research and intelligence.
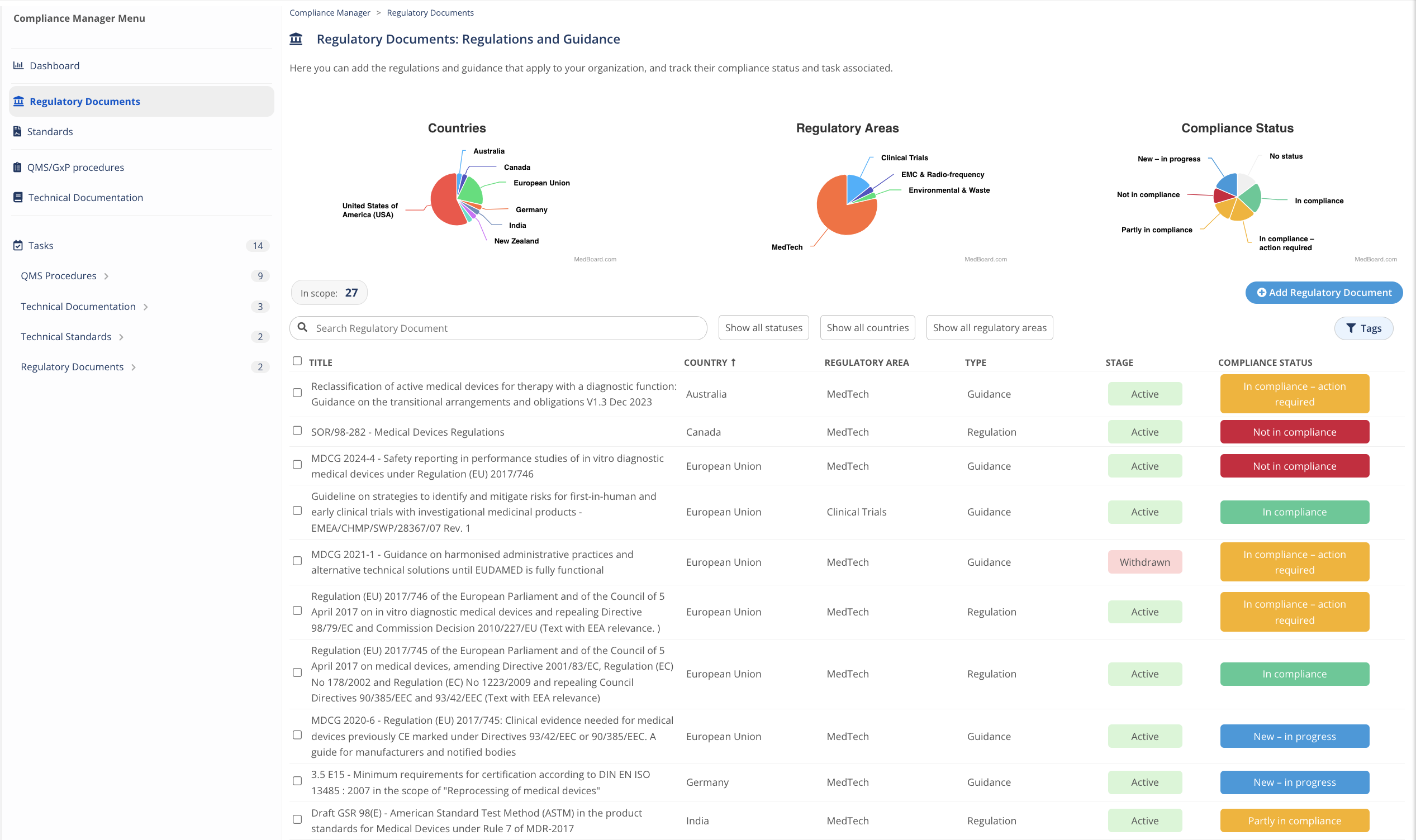
Add Documents and Standard that apply to your organization to the Compliance Manager!
![]()
Review systematically Regulatory News and Updates connected to our Global Data covering 225+ countries and 15+ regulatory areas in real time. Customize it, create protocols, impact assessments, actions, and reports easily with MedBoard.
![]()
Organize, manage and track your compliance evidence with the Compliance Manager. This module helps to identify and control the specific regulations, guidance, standards, procedures, technical documentation that apply to you, and to organize, action, and track your related compliance evidence.
![]()
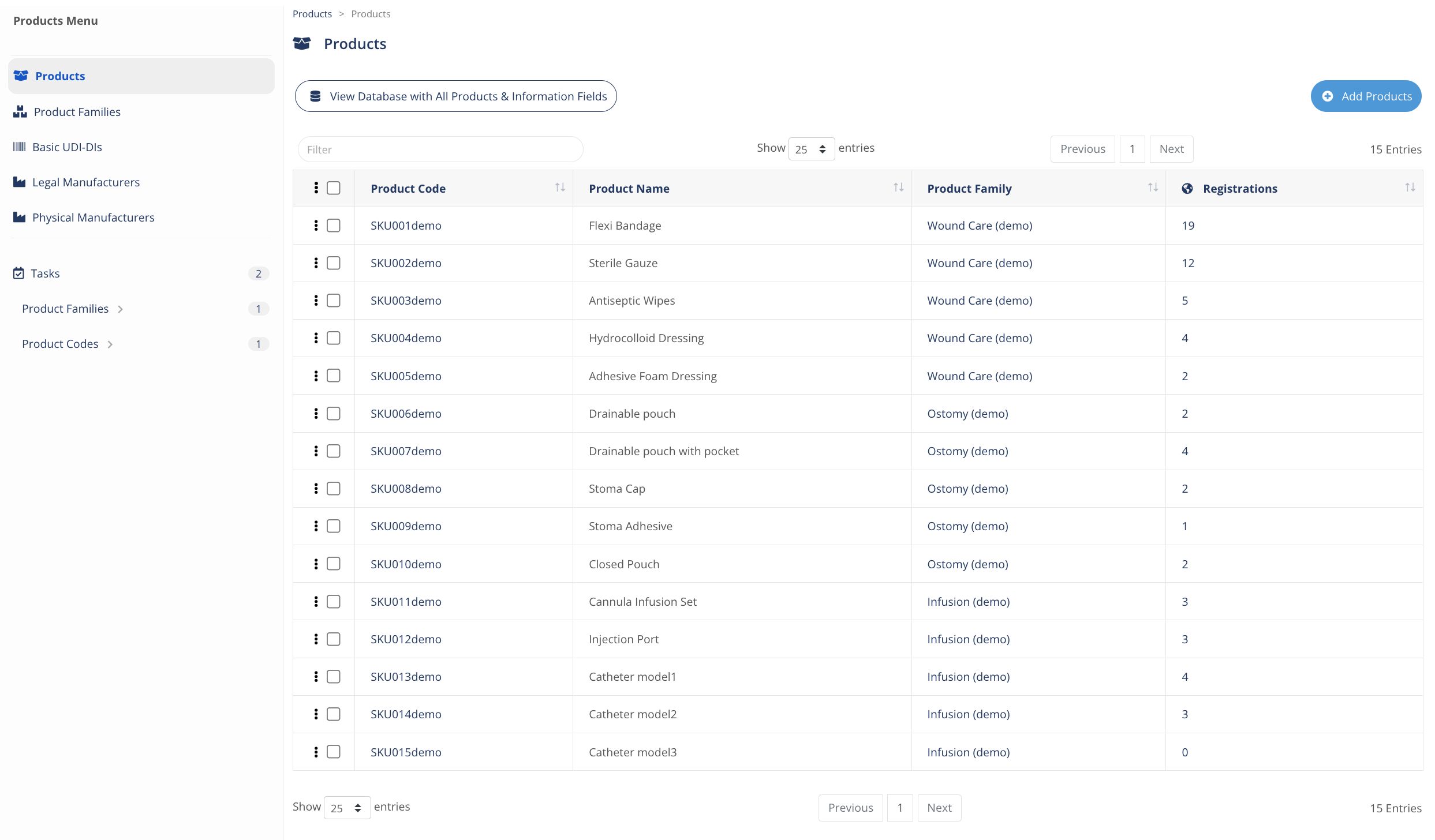
A powerful ready-to-use Products Information Management to organize, manage and track information about your products, product codes, SKUs, and its information, including Unique Identifiers (e.g. UDI), all integrated together with Regulatory Intelligence and MedBoard Search.
![]()
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, licenses and economic operators, all integrated together with Regulatory Intelligence, Regulatory Reviews, Task Manager and MedBoard Search.
All modules integrate seamlessly with each other,
to keep all connected, in one place.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams
