Clinical & Research Solutions
One Platform
for Clinical Research, Systematic Reviews and Evidence Management.
Solutions for each step of an effective Clinical Research and Compliance in a unified platform that provides end-to-end data management
Global data access
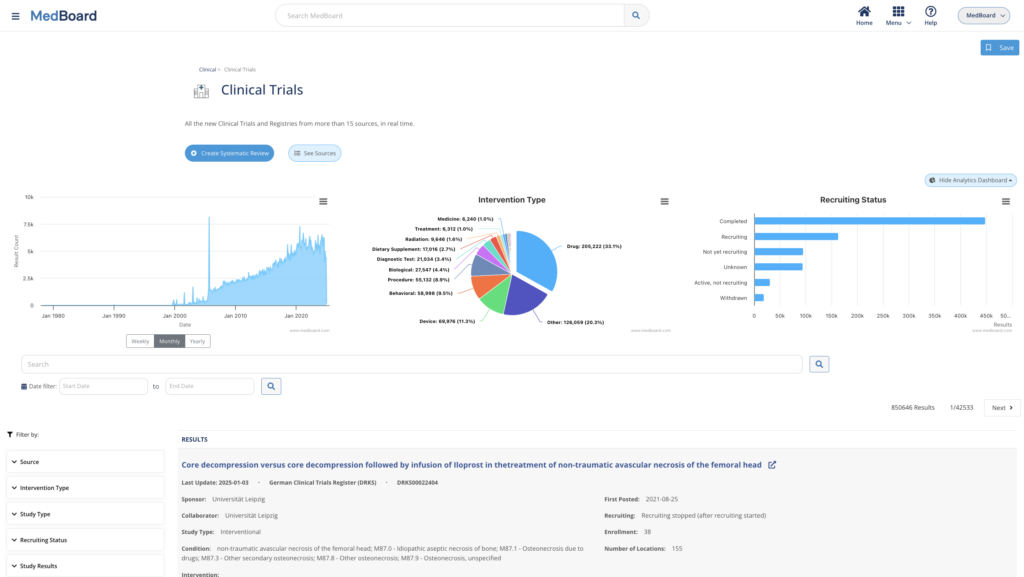
Large and curated AI-powered Databases with filters, analytics, and more
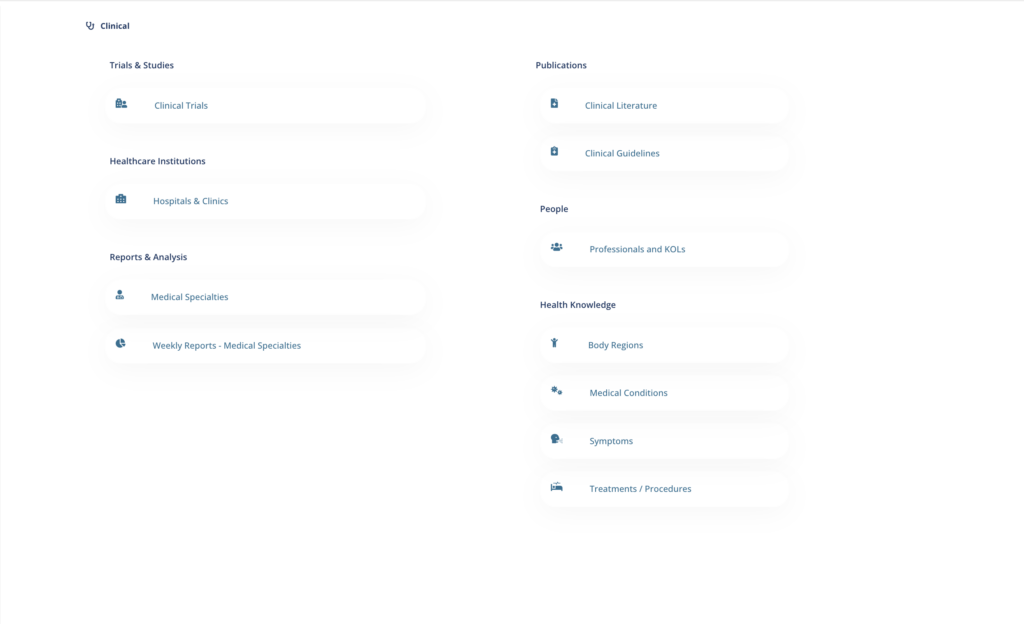
to access to up to date Clinical Databases and Information, including:
- Clinical Trials & Studies
- Clinical Literature
- Clinical Guidelines
- Hospitals and Clinics
- Professionals & KOLs
- Medical Conditions, Procedures, and Treatments
- Clinical news & Documents
Manage Literature Reviews
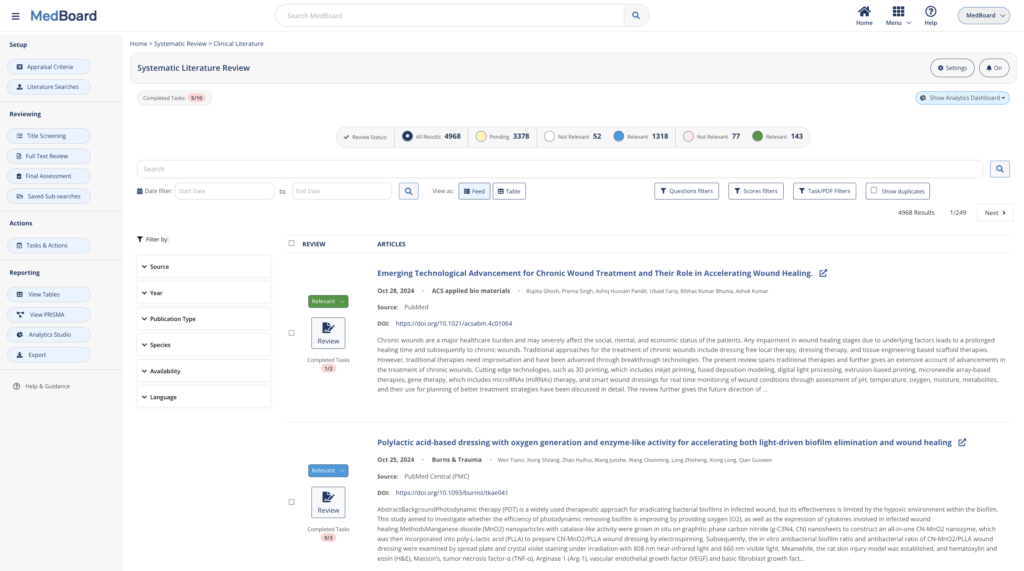
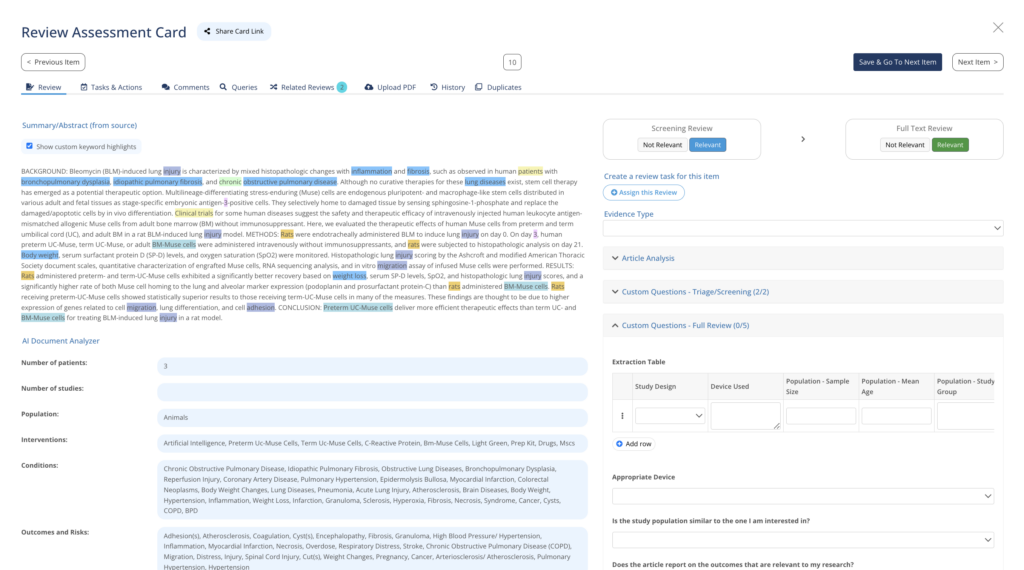
Perform Systematic Literature Reviews at lightning speed while keeping control of every step of the process and evidence.
Use MedBoard automations, workflows, integrated intelligence, and AI-powered features, to produce compliant systematic reviews faster, with more precision and more cost-effective.
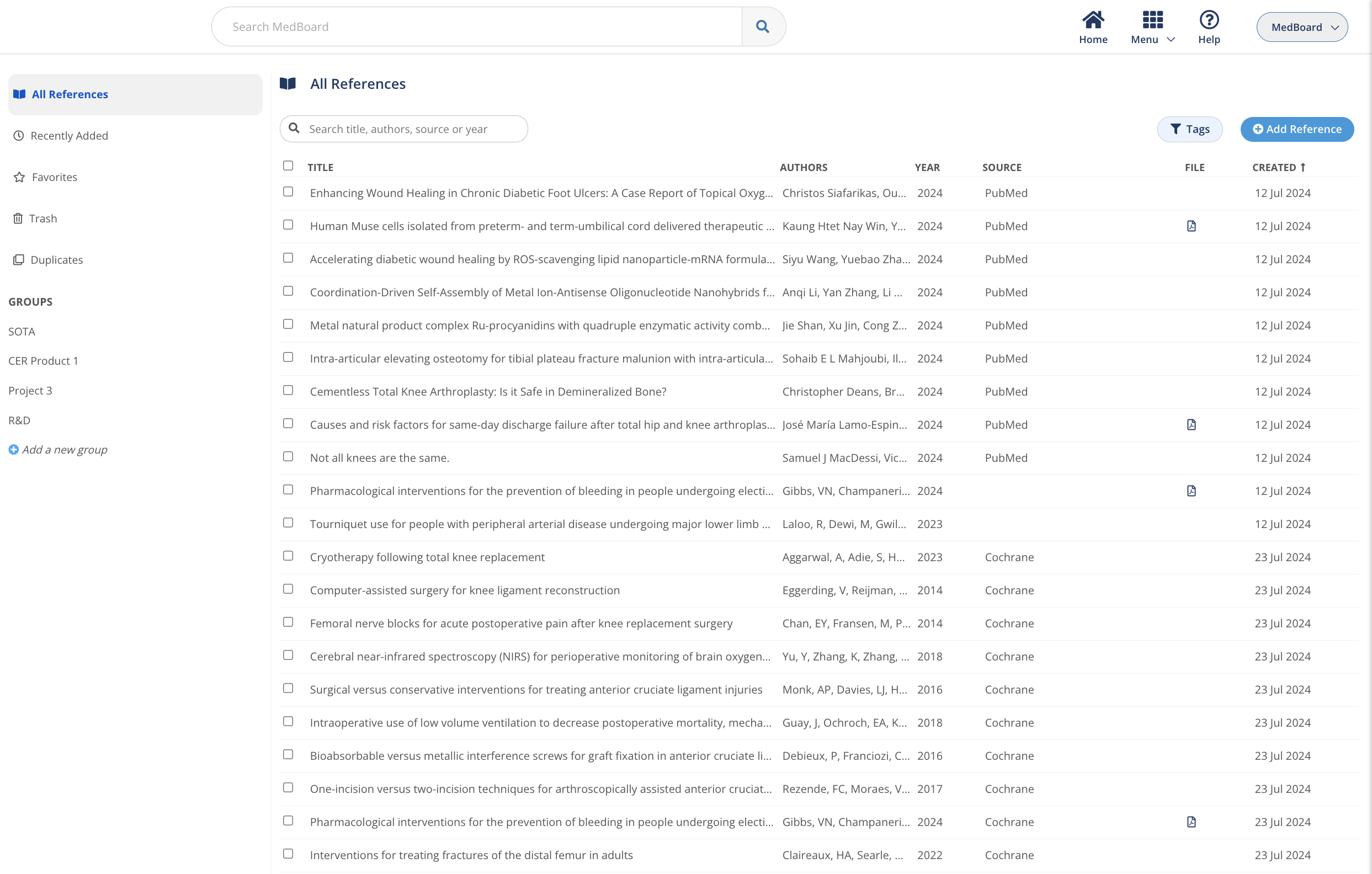
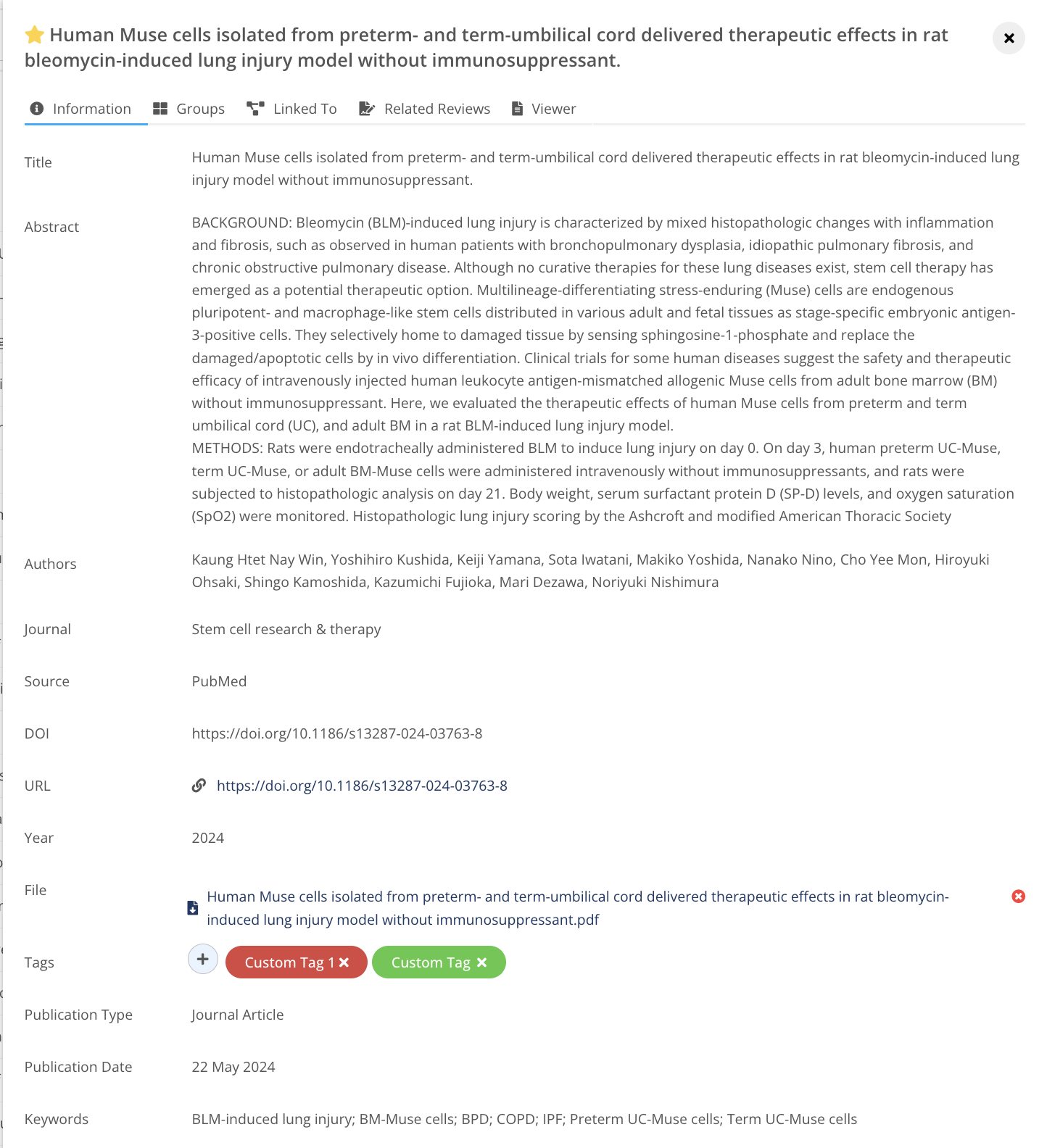
Manage references
Collect, manage, organize and access all your references and PDFs,
from only one place.
Seamless evidence management in a secure repository connected to
your key projects, processes and workflows within the MedBoard platform.

Manage Citations
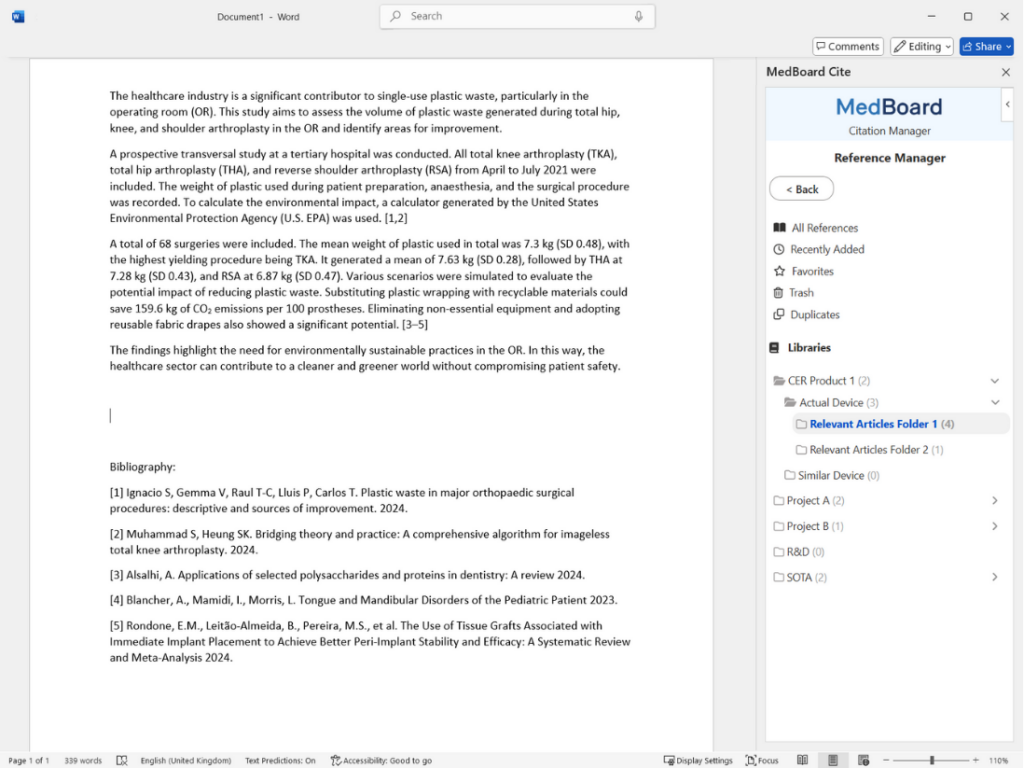
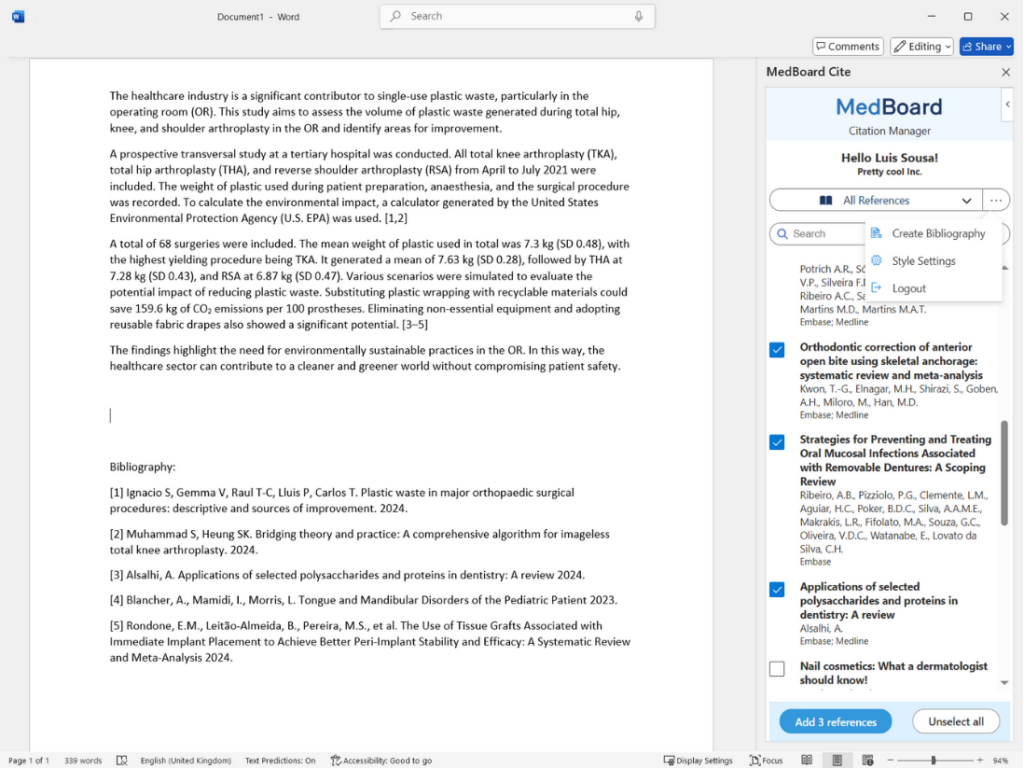
Add citations and bibliographies automatically from your libraries and folders within your MedBoard Reference Manager as you write in Microsoft Word.
Streamline your writing and referencing with MedBoard Cite, the powerful citation manager seamlessly integrated with MedBoard Reference Manager and Microsoft Word.
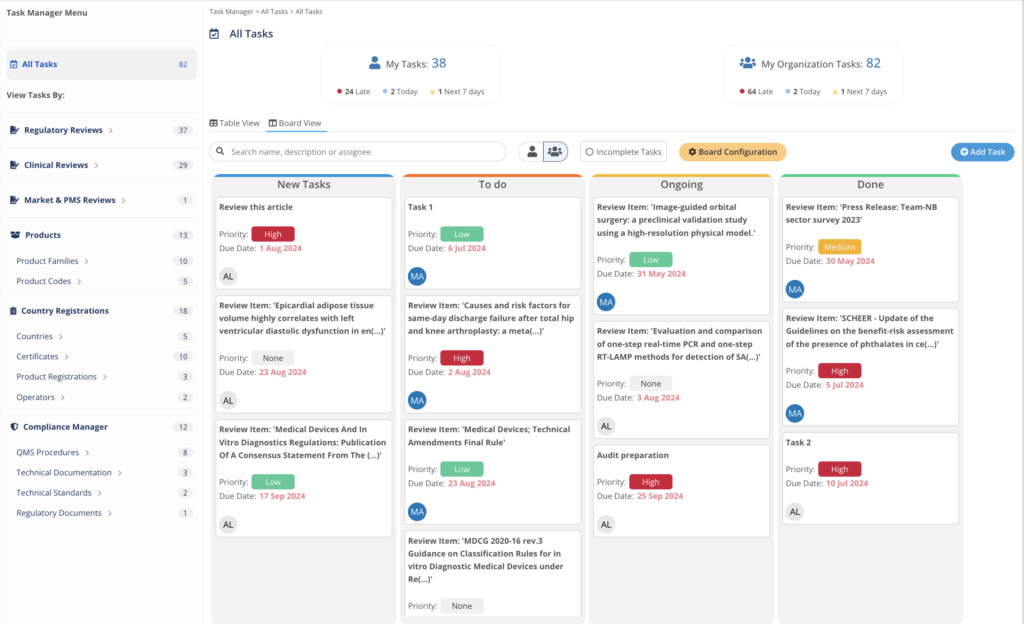
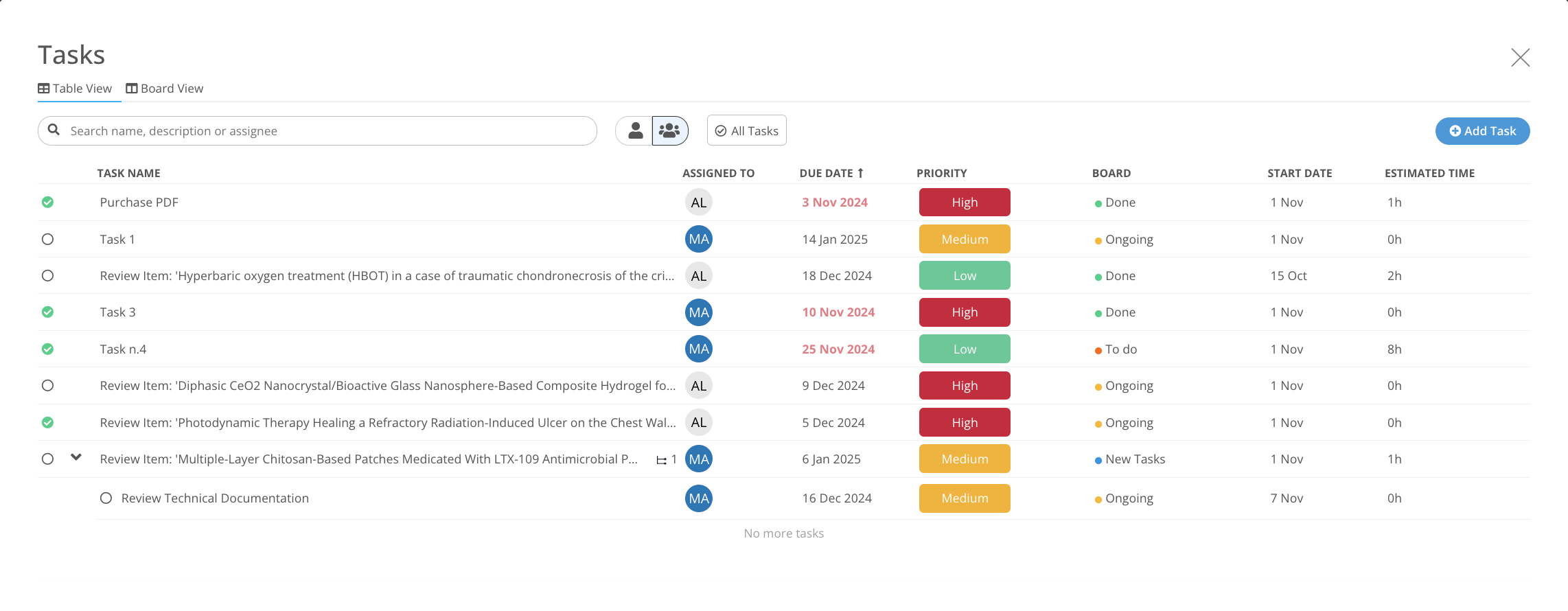
Manage work and deliverables
Keep Control over the process.
Task Manager integration in each MedBoard solution for Actions and Implementation.
- Create Tasks, Actions linked to Evidence, Articles and Products
- Deadlines and Responsible Person
- Email Notifications and Alerts
- Dashboard to report progress, and risk levels
100%
CSAT
Customer Satisfaction (CSAT) Score,
with an average overall satisfaction with MedBoard of 4,8/5.
50%
Reduced Manual Work
86% of MedBoard users report reducing
by more than 50% the need for
manual actions and work.
3x-10x
Faster Processes
78% of MedBoard users reported
performing actions 3x to 10x faster,
accelerating their processes.
Go Beyond: MedBoard platform offers even more
Discover all our connected solutions
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your evidence journey



