
Compliance Manager
Create your compliance register, and organize and track your compliance status globally and by region, instantly.
List all the regulations, guidance, technical standards, technical documentation and much more within your scope, in any region, and track their compliance status easily.
Integrated
Seamlessly integrated with MedBoard Intelligence and MedBoard regulatory solutions, including your regulatory news reviews.
Easy to use
Easily build and maintain the list of regulatory documents, standards, technical documentation and many more.
Connected
Connect your compliance with your evidence and products, technical documentation and certificates.
Customizable
Monitor compliance through visual dashboards, countries, regulatory areas, and custom tags.
Secure
Your Privacy and Security are our top priority. MedBoard is ISO 27001 certified, ensuring robust information security management.
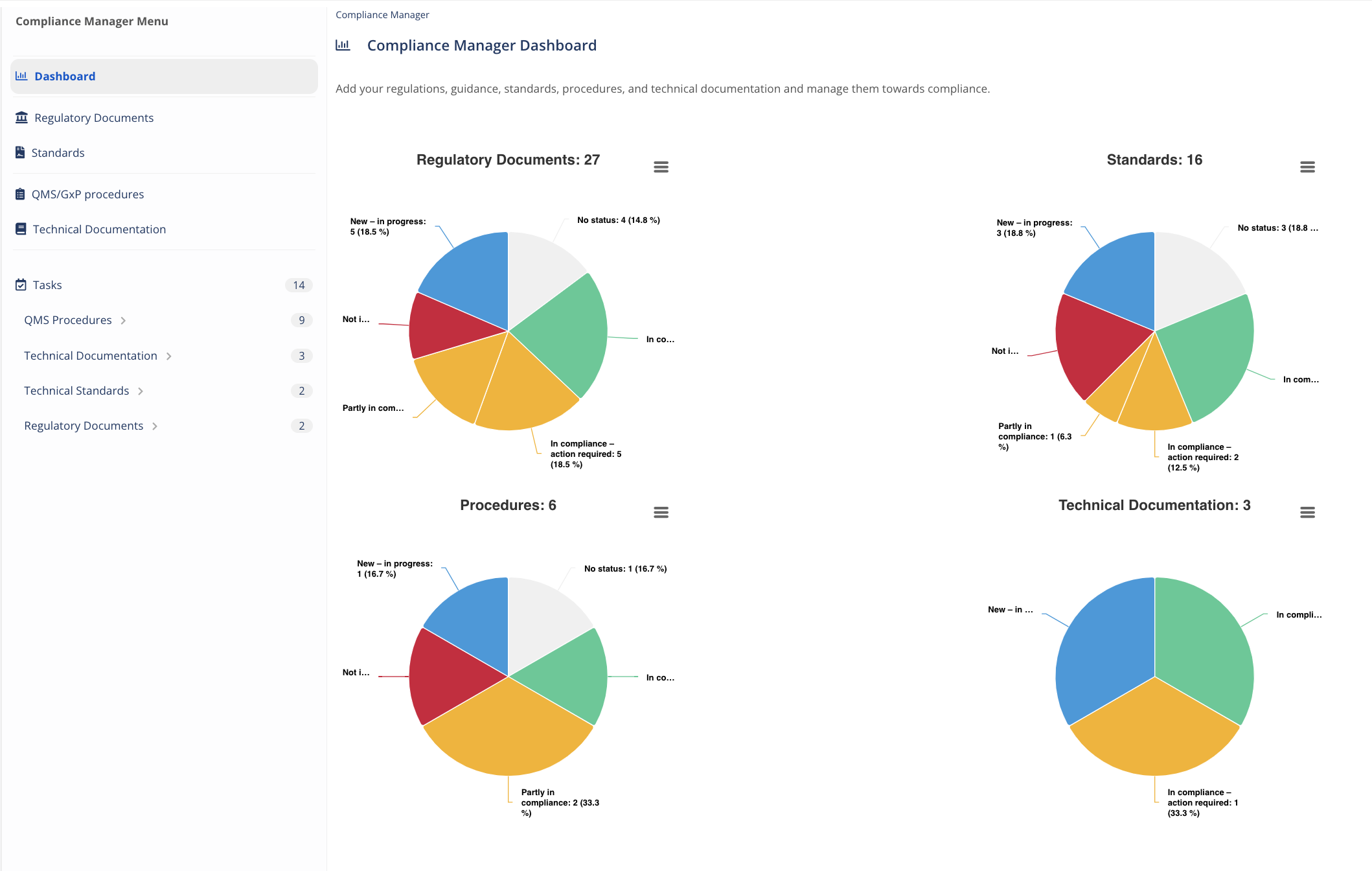
How many regulations and guidance are applicable to you right now and what is your compliance status?
If you cannot answer directly this question, you probably need Compliance Manager
Have full control over your compliance scope
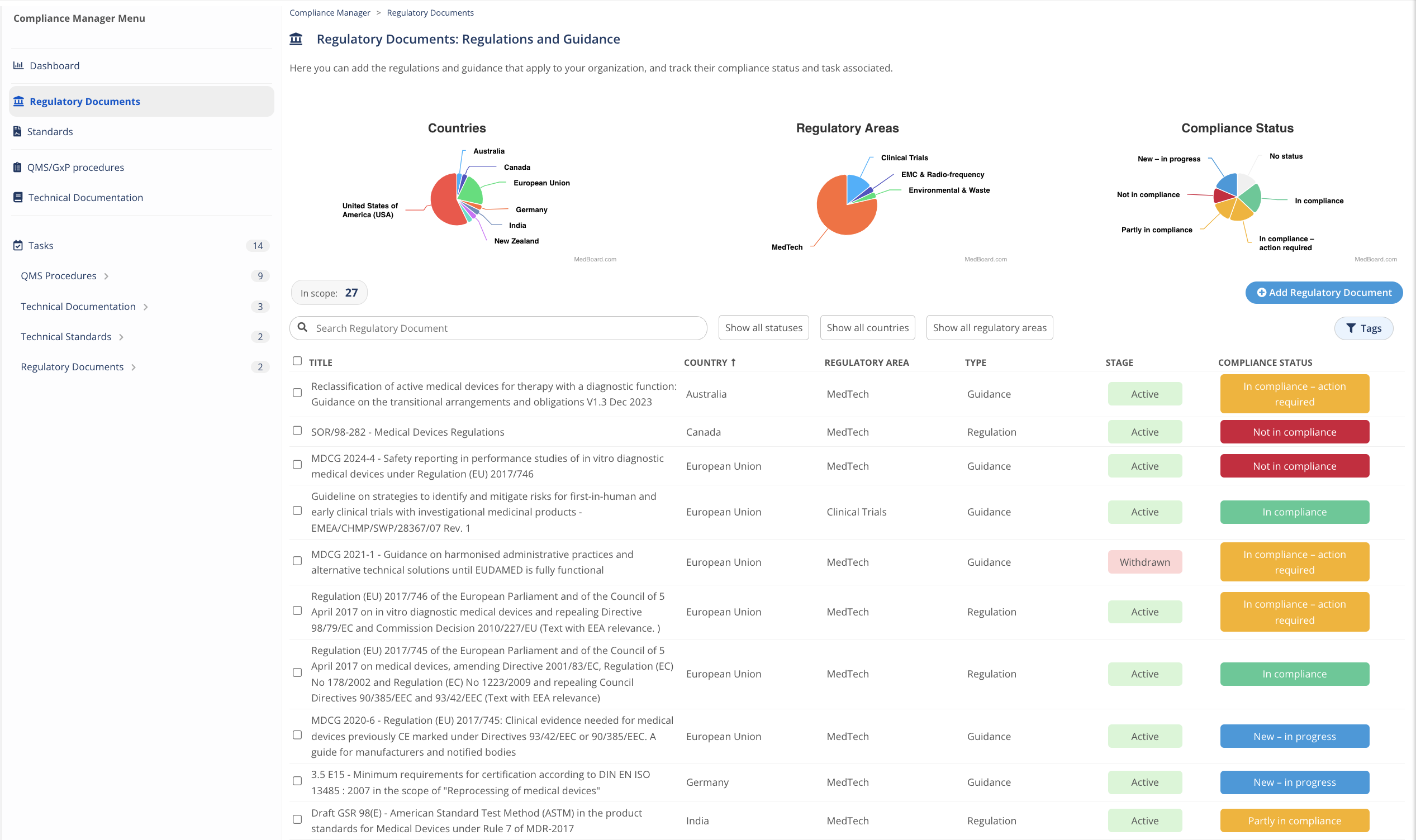
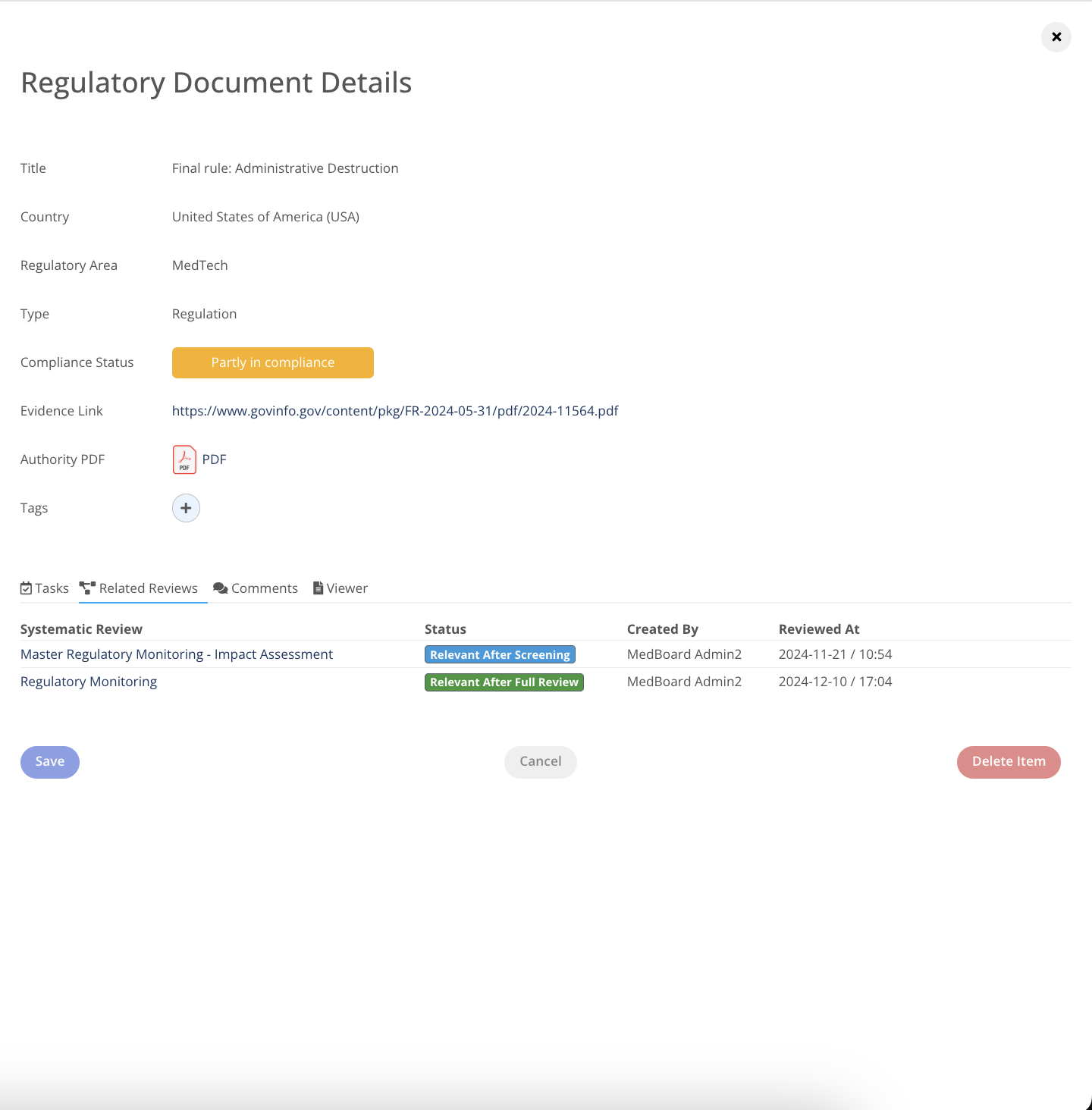
Add Documents and Technical Standards
Add Regulatory Documents and Technical Standards that fall within your scope of operations directly from MedBoard databases and reviews.
Add Technical Documentation
Add Technical Documentation and track their compliance, and link regulations, guidance, standards and actions to documentation.
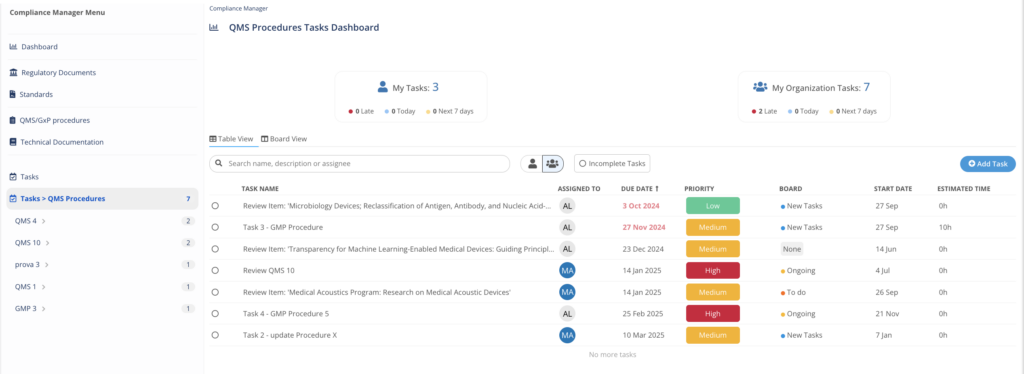
Add Procedures and processes
Add QMS/GxP Procedures and track their compliance, to link regulations, guidance, standards to impacted processes and track actions.
Report compliance by region instantly
Monitor, filter, and export documents within the scope by Compliance Status, by Country, by Regulatory Area, and by Custom Tags.
Customize your data
Add notes, comments, and custom tags to each document to customize the repository and reporting.
Connected to MedBoard Intelligence
The Compliance Manager is seamlessly integrated with MedBoard Regulatory Global Data, informing you of any status change of each document in your compliance portfolio.
MedBoard integrations and automations for full traceability
MedBoard Regulatory Reviews
Assess when a document was reviewed and the outcome. Seamlessly integrated with your impact assessment process, each document will be linked to your MedBoard Regulatory Reviews.
MedBoard PIMS / RIMS
Link Documents and Standards to your Product Codes, Product Families, Country Registrations, Operators and more from the integrated MedBoard RIMS.
MedBoard Task Manager
An integrated project management solution to keep track of required action, collaborate, and create tasks and deadlines. Tasks will be linked to each Document, Standard, Technical Documentation and Procedure.

Make the Most out of MedBoard
Discover our Software Products for Regulatory Needs and Professionals:
![]()
Access to up to date Regulatory Intelligence, data, tools and News in more than 225 Countries, organized, curated and cleaned by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma/BioTech and Clinical trials, that includes countries curated summaries for an unparallel research and intelligence.
Add Documents and Standard that apply to your organization to the Compliance Manager!
![]()
Review systematically Regulatory News and Updates connected to our Global Data covering 225+ countries and 15+ regulatory areas in real time. Customize it, create protocols, impact assessments, actions, and reports easily with MedBoard.
![]()
Organize, manage and track your compliance evidence with the Compliance Manager. This module helps to identify and control the specific regulations, guidance, standards, procedures, technical documentation that apply to you, and to organize, action, and track your related compliance evidence.
![]()
A powerful ready-to-use Products Information Management to organize, manage and track information about your products, product codes, SKUs, and its information, including Unique Identifiers (e.g. UDI), all integrated together with Regulatory Intelligence and MedBoard Search.
![]()
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, licenses and economic operators, all integrated together with Regulatory Intelligence, Regulatory Reviews, Task Manager and MedBoard Search.
All modules integrate seamlessly with each other,
to keep all connected, in one place.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams
