The AI-powered Compliance and Intelligence Automation Platform
The All-in-One MedBoard platform for Regulatory, Clinical, Market and PMS teams. Monitor real-time changes, manage evidence and intelligence, and automate compliance workflows in one platform, powered by global data and AI. Simplify, integrate and increase productivity and control with MedBoard.
Automation and knowledge platform for MedTech, Pharma, and Life Sciences
150+ Organizations rely on us (and growing every month!)
Trusted by many leading and innovative organizations, from large enterprises to start-ups
Solutions for every team. Powered by one platform.
Automate, simplify and unify your processes
3x-10x
Faster Processes
MedBoard users report
performing 3x to 10x faster,
accelerating their work massively.
50%
Reduced Manual Work
MedBoard users report reducing
by more than 50% the need for
manual actions and work.
100%
CSAT
Customer Satisfaction (CSAT) Score,
with an average overall satisfaction with MedBoard of 4,8/5.
Many solutions unified in one modular platform, choose yours:

Your research, intelligence and compliance on one unified platform
Regulatory | Clinical | Market | PMS & Vigilance
One big data platform, and multiple software products for all your needs.
Our unique platform approach

Global Knowledge platform
MedBoard data platform is huge, and we mean it, it is actually very huge! (over 1 Billion connected datapoints), providing global access to up to date information, data and knowledge, in Regulatory, Clinical and Market & PMS, with multiple databases and AI assistance.
MedBoard Search provides continuous and instant access in real-time to information and data, using only trusted sources, covering latest news, databases, intelligence, profiles, analytics, tools, documents, webpages, summaries, translations and much more.
The MedBoard data platform keeps updating in real-time and growing every day! Stay connected with what is happening.
Regulatory solutions
MedBoard platform is all it takes to transform your Regulatory Intelligence and Operations:
- Global Regulatory Databases.
- Regulatory Intelligence.
- Compliance Manager.
- Products Information Management.
- Country Registrations Management / RIMS.
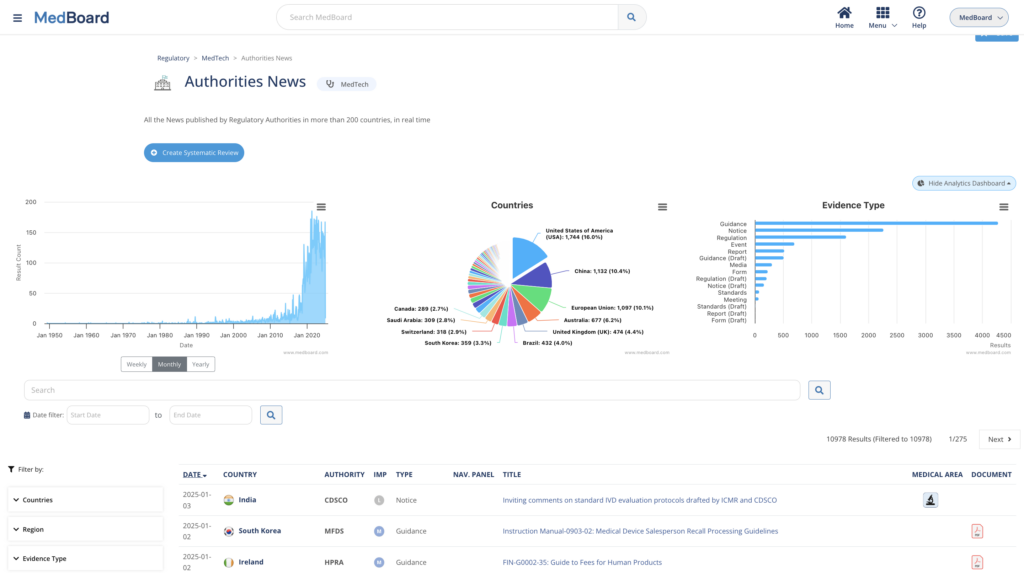
Real time data from 225+ Countries and 15+ Regulatory Areas (e.g., MedTech, Pharma, AI, Clinical Trials, etc.), integrated with powerful software solutions.
Use MedBoard Regulatory Solutions for each step of an effective Regulatory System in a unified platform that provides end-to-end data management.
Clinical solutions
MedBoard platform for Clinical Research, writing and compliance:
- Clinical Research Databases.
- Systematic Literature Reviews (SLRs).
- Reference Manager.
- Citation Manager for MS Word.
From large clinical databases, clinical trials and knowledge directories, integrated with research software solutions, for all-in-one researching, writing, annotation, and evidence workspace.
See MedBoard Clinical solutions for each step of an effective Clinical Research and Compliance in a unified platform that provides ent-to-end data management.
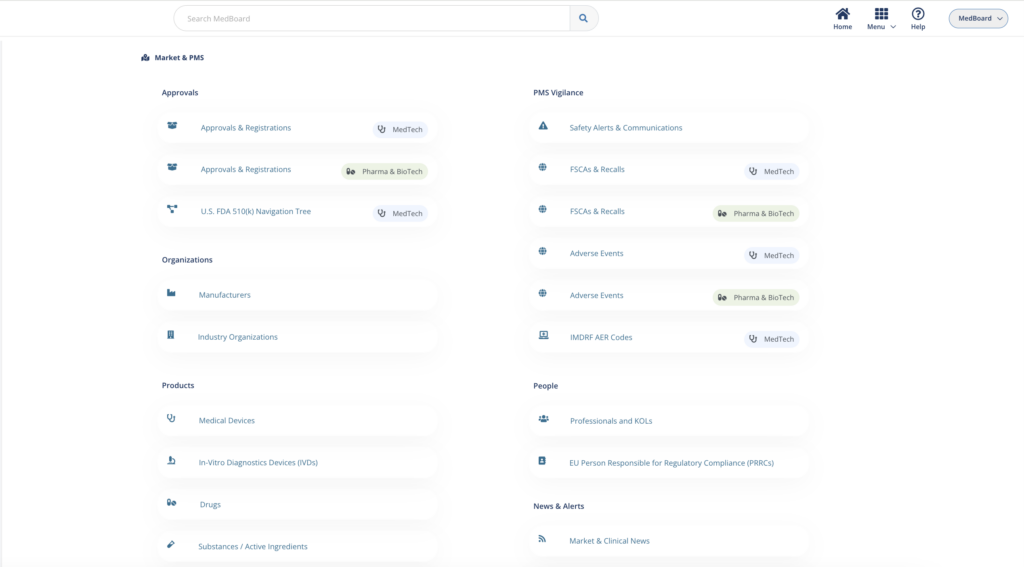
Market, PMS & Vigilance solutions
MedBoard platform for Market Research, Intelligence, PMS and Vigilance.
Market news, approvals, large market databases, vigilance databases (alerts, recalls, adverse events), profiles of products, manufacturers, KOLs, integrated with powerful software solutions:
- Market News and Approvals Monitoring and Reporting.
- PMS/PV Vigilance Databases Monitoring and Reporting.
- Market Lists
Use MedBoard Market, PMS & Vigilance Solutions for each step of an effective Market Research, Intelligence, and Vigilance system.


Work management
MedBoard platform for work, projects and tasks management.
Take control over all the deliverables, evidence, deadlines and responsibilities, integrated with any process within the platform:
- Project & Task Manager
Use Work Management Solutions to effectively plan, monitor and implement your individual and team work.
Security and Trust
Your security and privacy is our priority

Loved by our customers
Why Organizations choose MedBoard?
MedBoard unique approach provides instant benefits:

Global Information Access
Unparalleled and real-time access to global data for regulatory, clinical and market, with multiple number of advanced databases and tools.

Productivity Increase
Our platform allows teams to fully customize their work and increase their efficiency massively in processes and access to information.

Time & Costs Savings
Automate, digitize and reduce extraordinary amount of work (mostly manual), with labour, repetitive tasks and processes.

Easy to Use
Customers report 'Easy to Use' and friendly UI as one of the top reasons to use MedBoard, we are transforming complex processes into simple steps.

Multiple Solutions
MedBoard is a multi-solution platform, providing many solutions in just one place, with a unique approach of data plus sotware solutions.

Trusted & Proven Value
Security, compliance and privacy built in features, including SSO. Our platform is also validated with a proven ROI from customers.
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your work
Global Data
MedBoard Search, News & Databases
MedBoard Global Data and Intelligence Platform is huge, we mean, it is actually very huge! (over 1 Billion connected datapoints), providing global access to up to date information, data and knowledge.
MedBoard only uses trusted sources, and provides continuous and instant access in real-time to information and data, covering News, Databases, Intelligence, Profiles, Analytics, Tools, Documents, Webpages, Summaries, Translations and much more.
The MedBoard platform keeps updating in real-time and growing every day!
Stay connected with what is happening
Regulatory Global Data
In more than 225 countries, access to up to date Regulatory Intelligence, data, tools and News, organized, classified and curated by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma, BioTech and Clinical Trials, that includes countries curated summaries for an unparallel research and intelligence.
Clinical Global Data
Access to up to date Clinical databases and information, including Clinical Trials & Studies, Literature, Guidelines, Hospitals and Clinics, professionals & KOLs, medical conditions and treatments, clinical news and much more.
Integrated with advanced filters, analytics and integrations with other MedBoard databases, for a faster clinical research and intelligence.
Market & PMS Global Data
Access to up to date Market Databases including Approvals, Medical Products profiles (Devices, IVDs, Drugs, Substances, Apps), Medical Manufacturers, KOLs, market news and much more.
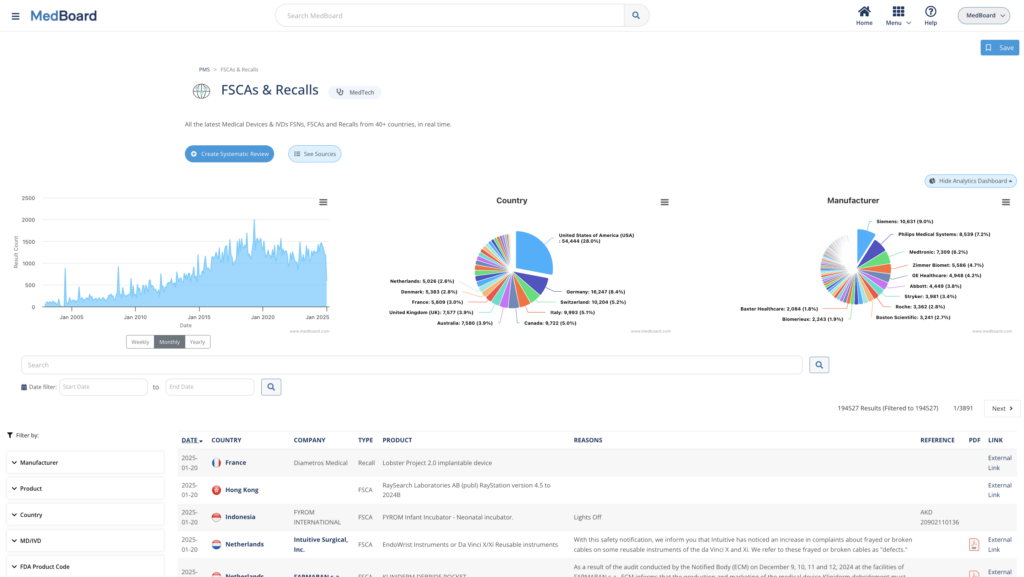
PMS Vigilance Databases include Safety Alerts, FSCAs & Recalls, Adverse Events and Shortages. Integrated as always with advanced features and data.
“MedBoard is impressive, nothing compares to it”
Software Products
Multiple software products to choose from and combine depending on your needs
Regulatory & Compliance products
SRs: Regulatory News
Review systematically Regulatory News and Updates connected to our Regulatory Global Data covering 225+ countries and 15+ regulatory areas in real time. Customize reviews, create workflows, impact assessments, actions, and reports easily with MedBoard.
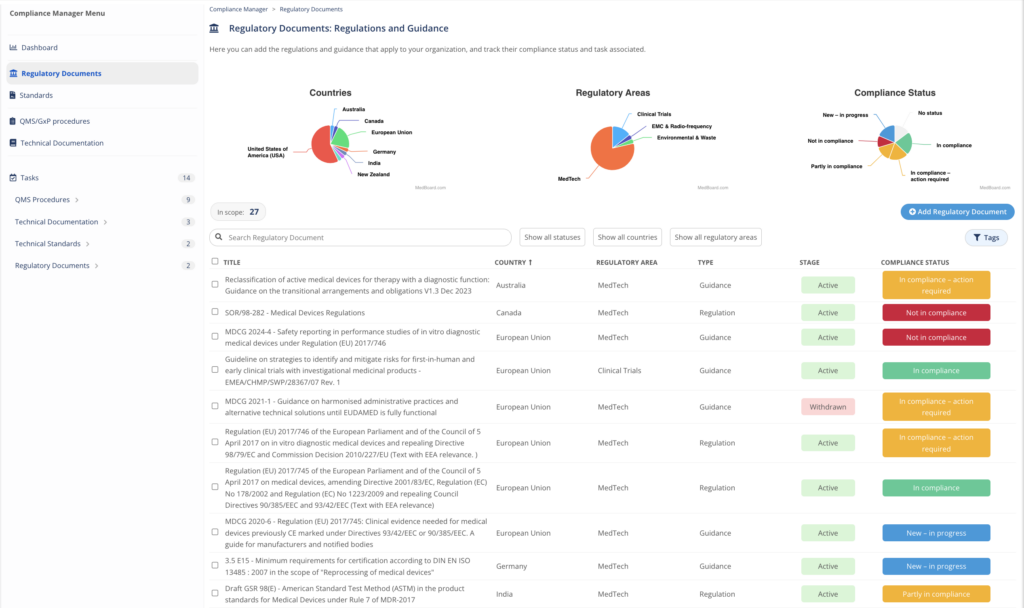
Compliance Manager
Organize, manage and track your compliance evidence with the Compliance Manager. This module helps to identify and control the specific regulations, guidance, standards, procedures, technical documentation that apply to you, and to organize, action, and track your related compliance evidence.
Products Information
A powerful ready-to-use Products Information Management to organize, manage and track information about your products, product codes, SKUs, and its information, including Unique Identifiers and claims, all integrated together with Regulatory Intelligence and MedBoard Search.
Country Registrations RIMS
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, licenses and economic operators, all integrated together with Regulatory Intelligence, Regulatory Reviews, Task Manager and MedBoard Search.
Clinical products
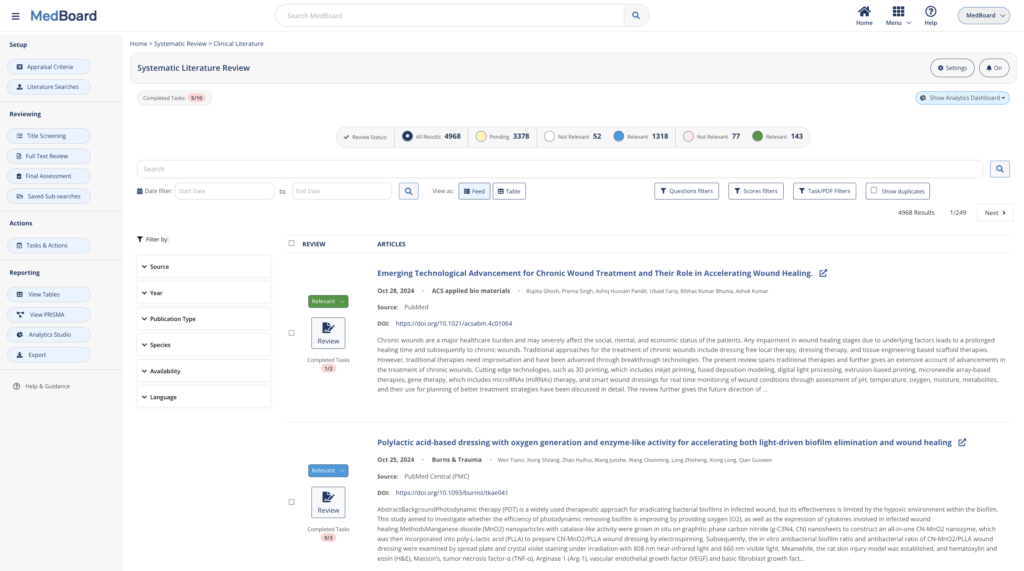
Systematic Literature Reviews
Review systematically Literature and accelerate your systematic review workflow from search to reporting. Whether you import automatically results or import results from your own databases, MedBoard software and AI-features will simplify and customize to your needs the process, search, review and reporting.
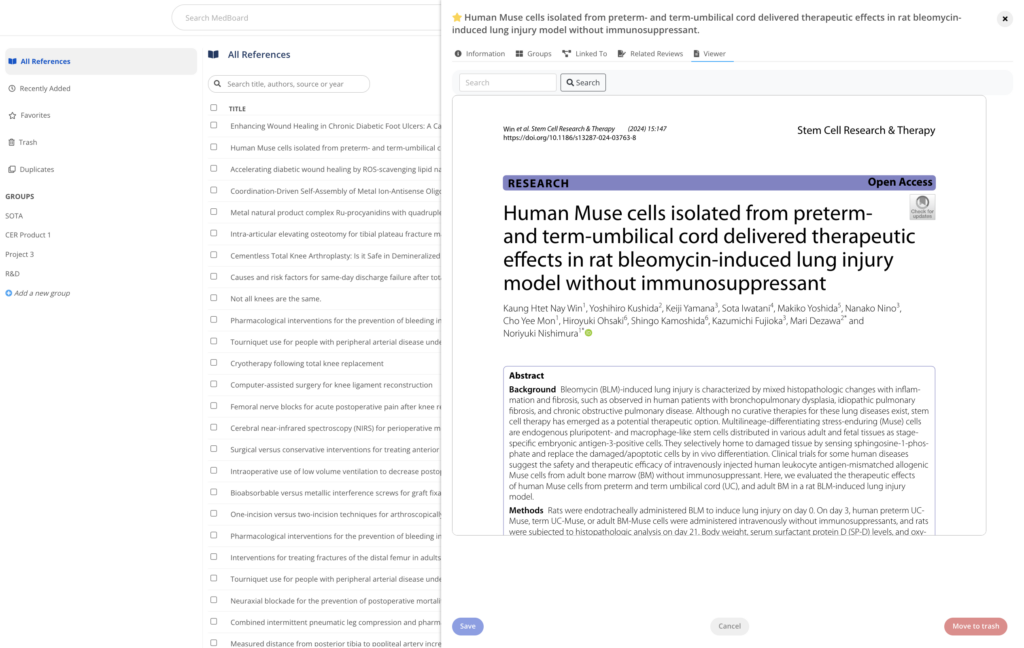
Reference Manager
A reference management software to manage your references, bibliography and PDFs. Including features such as duplicates and custom tags, and seamlessly integrated with your MedBoard Literature Systematic Reviews, MedBoard products and data, to keep all your clinical evidence connected in one place.
Market & Vigilance products
SRs: Market & PMS
PMS Adverse Events, Recalls, FSCAs, Safety Alerts, Approvals and Registrations, press releases, media news, and many more databases where to choose to perform Market, Post-Market Surveillance (PMS) and Vigilance monitoring.
Market Lists
Create custom lists with Manufacturers, Products, Substances, Hospitals, Researchers, KOLs, and manny more types from the 10M+ MedBoard Profiles vast collection to analyse changes in statistics/analytics by custom date periods, add intelligence, tags, notes, reviews, and much more in only one place.
Work Management products
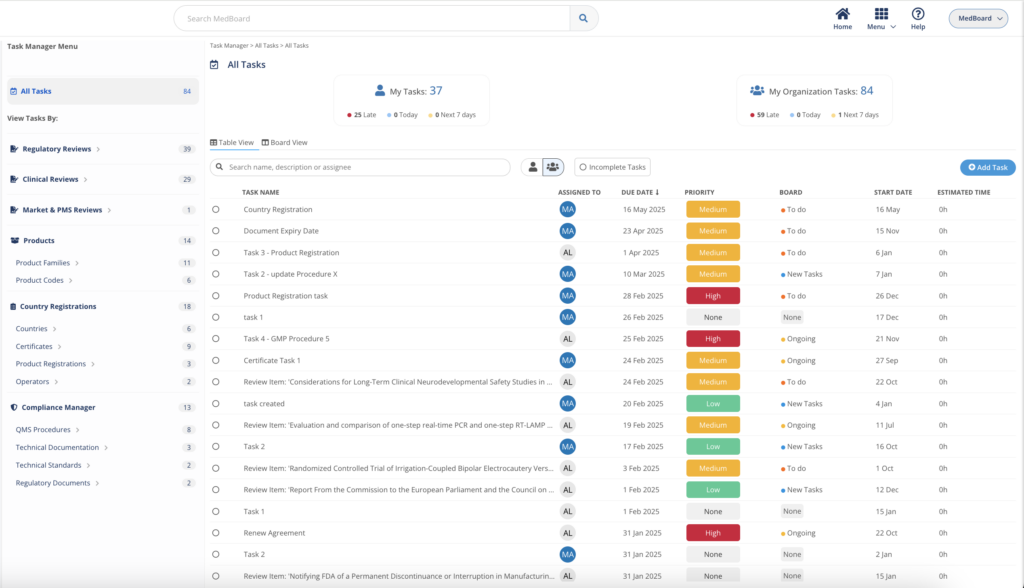
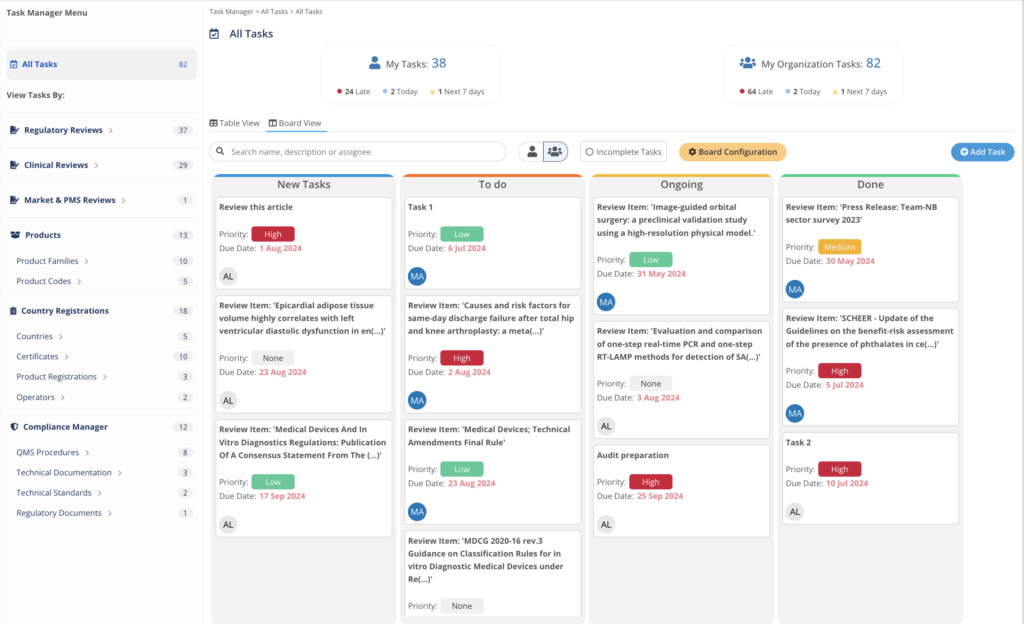
Task Manager
The Task Manager integrates with MedBoard data and modules to easily action, track, and complete efficiently any task and project by teams and professionals. Customize your workflow, and view your projects as a Task List or Board View. Easy to use, with intuitive UI to help you get started fast.
Increase productivity, collaboration, transparency and communication across your organization
Our customers report a 5x increase or more since using MedBoard
Seamless integration between Global Data and Software Products
Flexible approach to build processes and workflows, easy to implement
Implementing processes within your QMS or QxPs? No problem! we can also support with ready-to-use software validations, training, and work instructions.